Dopamine derived copper nanocrystals used as an efficient sensing, catalysis and antibacterial agent†
Abstract
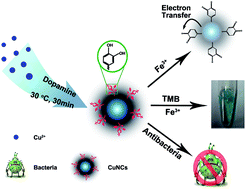
A simple one-pot synthesis method for small copper nanocrystals (CuNCs) was developed by employing dopamine as a reducing and capping reagent. The as-prepared CuNCs exhibited a fluorescence emission at 390 nm, good peroxidase-mimicking catalytic property, and excellent antibacterial activities against Gram-positive Staphylococcus aureus. Based on the fluorescence and peroxidase-mimicking catalytic features, sensing for ferric ions (Fe3+) was made because Fe3+ ions have specific interactions with the catechol groups on the surface of CuNCs with the limits of detection of 1.2 μM and 4.2 μM, respectively, which were much lower than the maximum level (5.4 μM) of Fe3+ permitted in drinking water by the US Environmental Protection Agency. For the antibacterial activities, a minimum inhibitory concentration of 158 μg mL−1 was found which was because of the generation of reactive oxygen species.

 Please wait while we load your content...
Please wait while we load your content...