Graphitized porous carbon prepared from pyrolysis of Sterculia scaphigera and its application in lithium ion batteries†
W. X. Wanga,
Y. Wana,
S. F. Wua,
M. C. Lia,
L. J. Caoa,
F. C. Lva,
M. Y. Yanga,
Z. F. Suna,
R. Sunb and
Z. G. Lu*a
aDepartment of Material Science and Engineering, South University of Science and Technology of China, Shenzhen, Guangdong 518055, China. E-mail: luzg@sustc.edu.cn
bShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, Guangdong 518055, China
First published on 11th May 2015
Abstract
Sterculia scaphigera exhibits exceptional capability to inhale a large amount of water, which is accompanied by great volume expansion. In this study, we present, for the first time, the eco-friendly preparation of graphitic porous carbon materials via a simple pyrolysis of H2O-adsorbed Sterculia scaphigera under moderate temperature. Nitrogen adsorption/desorption, X-ray diffraction, Raman spectra and transmission electron microscopy characterizations indicate that water adsorption plays a critical role in developing the abundant micropore architectures and high specific surface area as well as promoting the graphitization degree of the as-obtained porous carbon. Furthermore, the as-prepared porous carbon demonstrated superior electrochemical performance with a good combination of moderated capacity, good rate capability, extremely stable cycling and high coulombic efficiency.
1. Introduction
With the depletion of fossil fuel, the exploitation of green and sustainable energy has become increasingly important, which causes the introduction and rapid development of large-scale energy storage devices.1,2 In particular, the rechargeable lithium ion batteries (LIB) are one of the most attractive renewable devices because they have successfully driven the wide commercialization of potable electronics and applications in electric vehicles.3 However, the development of LIB largely lags behind the progress of consumable electronics and the successful application of LIB in electric vehicles needs further exploration. In particular, the lack of suitable anode material with appropriate comprehensive electrochemical performance is the bottleneck.In recent years, various nanostructured Si,4 Sn,5 and some types of transition metal oxides6,7 with ultra-high theoretical capacities have been utilized as anode materials for LIB owing to their conversion reaction mechanisms for lithium storage. Nevertheless, graphitic carbon because of its low cost, easy processability, chemical stability, and desirable electrochemical profile8 has been overwhelmingly used for the anode material of LIB on the market. Inspired by graphite-based carbon, further exploration of novel carbon materials and composites with high capacity or desirable rate performance is underway.8–12 Soft carbon is a sort of graphitizable carbon, which shows both graphitic and non-graphitic structures.13,14 This type of carbon has been of particular interest because it has higher capacity than the theoretical capacity of traditional graphite and superior rate capability at high current density.14,15 Although several publications about soft carbon derived from petroleum pitch, coke and other organic precursor14–18 have been reported, its electrochemical performance is somewhat undesirable. Moreover, very few literature studies have investigated the electrochemical properties of soft carbon originating from natural biomass to date.
Herein, we reported a feasible, economic and eco-friendly route for preparing graphitized porous carbon from the natural biomass of Sterculia scaphigera for the first time. The spindle-shaped Sterculia scaphigera is mainly composed of bassorin, galactose and pentaglucose. When it is immersed into water, the natural Sterculia scaphigera gradually swells due to the adsorption of a large quantity of water. The swollen Sterculia scaphigera recovers to dry appearance after water evaporation. This process involves simple water inhalation and evaporation, eventually leading to the evolvement of highly porous structures. The as-obtained carbon is highly porous possessing good graphitic degree. Most importantly, no metal catalysts were used and the carbonized temperature was moderate (around 800 °C). Furthermore, no toxic gases were exhausted during the carbonization process. Therefore, this study introduces a green and economic concept, meanwhile providing a feasible approach for preparing graphitized porous carbon materials from natural biomass for application in LIB.
2. Experimental section
2.1 Preparation of graphitic porous carbon
The spindle-shaped Sterculia scaphigera (Fig. 1a) was first rinsed with deionized water several times to remove the impurity adsorbed on the surface. After that, whole Sterculia scaphigera was soaked in deionized hot-water (approximately 60°) for about 5 h so as to inhale enough water. In this process, water would be smoothly absorbed into Sterculia scaphigera, which triggers swelling and the development of pore structures, as illustrated in Fig. 1c and d. When Sterculia scaphigera inhaled adequate water and saturated, it was dried in the oven at 100 °C for 10 h. As shown in Fig. 1e, the dried Sterculia scaphigera resembles a thin film and is highly transparent. Thereafter, the dried products were subsequently annealed at high temperature. First, the temperature was increased from ambient temperature to 250 °C at a heating rate of 1 °C min−1 and held for 3 h in a pure argon (Ar) atmosphere. Second, the temperature was increased to 850 °C and kept for 6 h (the heating rate is 5 °C min) in Ar. After cooling to room temperature, the carbonized Sterculia scaphigera was finely ground and then washed with ethanol and distilled water several times to remove impurities. Finally, the sample was dried under a vacuum oven at 80 °C for 24 h. The resultant carbon samples derived from carbonized Sterculia scaphigera were denoted as SSC-X, where X represents the different carbonization temperatures. For comparison, another carbon was prepared at 800 °C by directly carbonizing natural Sterculia scaphigera without adsorbing water and designated as RSSC-800. The whole preparation procedure is schematically illustrated in Fig. 1.2.2 Characterizations
The crystallinity of carbonized samples were analyzed by Siemens X-ray generator with Cu Kα radiation (λ = 0.15406 nm) in the range of 10°–80°. Nitrogen adsorption/desorption isotherms were measured using a Micromeritics, ASAP2020 gas sorption analyzer at 77 K. All samples were degassed at 200 °C on a vacuum line following a standard protocol before measurement. The specific surface area was determined from N2 adsorption isotherm by applying Brunauer–Emmett–Teller (BET) theory and the pore size distributions were obtained from the adsorption branch by means of density function theory (DFT). The total pore volumes were determined from the amount adsorbed at the high relative pressure of 0.99. Graphitized degree of samples was also detected by Raman spectroscopy using Jobin-Yvon T6400 confocal Raman spectrometer with 532 nm diode laser excitation on 1800-line grating. The morphology of samples was characterized with field-emission scanning electron microscopy (FESEM, JEOL 7001F). High-resolution transmission electron microscopy (HRTEM) images were obtained from field-emission transmission electron microscopy (JEOL, JEM-2010F).2.3 Electrochemical test
For electrochemical measurements, 70 wt% active material, 20% acetylene, and 10% polyvinylidene difluoride (PVDF) were formulated by adding to N-methyl-2-pyrrolidone (NMP) and ground within agate mortar for 0.5 h to form homogeneous slurry. The slurry was then coated on copper foil uniformly and dried for 24 h at 80 °C in a vacuum oven. The loading of active materials was around 1 mg. The electrolyte was composed of a solution of 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (by volume) ethylene carbonate (EC)–diethylene carbonate (DEC). The sandwich cells were assembled in Ar-filled glove box (Mbraun, H2O and O2 < 0.1 ppm) by taking the cathode film and Li metal foils acting as anode. Cyclic voltammetry (CV) and impedance experiments were completed using Bio-Logic VMP2 multichannel potentiostat. Electrochemical impedance spectroscopic (EIS) profiles were recorded by applying an oscillating voltage of 5 mV over a frequency ranging from 10 mHz to 100 kHz. Galvanostatic charge/discharge that primarily tests the cycling life and rate performance under the voltage window 0.05–3 V was carried out using Arbin BT2000 multichannel testing equipment at room temperature.
1 (by volume) ethylene carbonate (EC)–diethylene carbonate (DEC). The sandwich cells were assembled in Ar-filled glove box (Mbraun, H2O and O2 < 0.1 ppm) by taking the cathode film and Li metal foils acting as anode. Cyclic voltammetry (CV) and impedance experiments were completed using Bio-Logic VMP2 multichannel potentiostat. Electrochemical impedance spectroscopic (EIS) profiles were recorded by applying an oscillating voltage of 5 mV over a frequency ranging from 10 mHz to 100 kHz. Galvanostatic charge/discharge that primarily tests the cycling life and rate performance under the voltage window 0.05–3 V was carried out using Arbin BT2000 multichannel testing equipment at room temperature.
3. Results and discussion
3.1 Structural features of the prepared materials
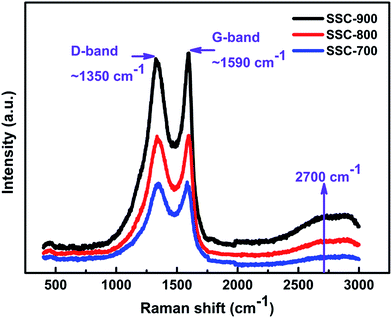
The structure of resultant sample carbonized with different temperature was analyzed by XRD. A collection of experimental XRD profiles for the four samples is shown in Fig. 2. All carbon samples show identical patterns, which are characteristic of 002 major peak at 2θ ∼ 27° marked as a typical of graphite like nano-crystallites and broad peak at higher angle 2θ ∼ 43° noted by the convolutions of (10) hk bi-dimensional line and the weak 004 reflection.19 As discussed by Inagaki,20 the appearance of hk lines is attributed to turbostratic stacking of hexagonal layers, which reflects the loss of regularity along the c-axis resulting in the formation of disordered carbon material. These XRD patterns match the features of soft carbon material reported in the literature.14,21 It is worth noting that the peak (002) slightly sharpens with increasing carbonization temperature, suggesting that the graphitization degree of as-prepared carbon is gradually enhanced.Further insight into the crystallinity of the carbon structure was investigated by Raman. Fig. 3 reveals typical Raman spectra of three carbon samples. These carbon samples exhibit two intense bands marked at 1350 and 1590 cm−1 assigned to the D (disorder carbon) and G (graphite carbon) bands of carbon materials, respectively.22–24 The relative peak height and width of the two carbon bands significantly vary as the temperature rises from 700 to 900 °C. In particular, the G band tends to shift toward higher frequencies and gets narrower when carbonization temperature goes up to 900 °C, revealing the increase of the interplanar (La) and interplanar (Lc) microcrystallite dimensions.23,24 The ratio of the two band intensity (IG/ID) ranges from 0.99 to 1.05 in Fig. 3. Interestingly, a broad and weak second-order band was observed at approximately 2700 cm−1 for the SSC-800 and SSC-900 samples. For the RSSC-800 sample, the intensity ratio is only 0.93, as shown in Fig. S1,† and no evident broad and second-order band emerges around 2700 cm−1. The variation of Raman spectra substantially indicates the increase of graphite degree through water assistance, further supporting the results observed in the XRD patterns. In addition, the graphitization degree of SSC-800 has been enhanced as compared with the RSSC-800 sample.
FESEM and TEM technologies were applied to characterize the microstructure of synthesized carbon materials. Fig. 4a shows the FESEM image of natural Sterculia scaphigera without any treatment. It can be seen that the natural Sterculia scaphigera mainly comprises smooth and wrinkled sheets several micrometers in size. After water adsorption and evaporation, the micromorphology of Sterculia scaphigera swells remarkably and evolves into lots of accumulated micro- and nano-particles on the surface of oval bulk. In addition, the surface looks rougher than that of raw Sterculia scaphigera, as seen from Fig. 4b. Moreover, the macromorphology of Sterculia scaphigera, shown in Fig. 1e, resembles a transparent thin film. As a result, it can be speculated that the effect of large amounts of water inhalation on the micromorphology is distinctly visible, and the thinner film helps to promote the graphitization of Sterculia scaphigera at high temperature. Fig. 4c exhibits a high magnification image of carbon sample carbonized at 800 °C in Ar. It reveals that the bulk sheet-like architecture is constructed by the agglomeration of nanoparticles. Meso/macropore structure has also developed from the accumulated nanoparticles. Fig. S2a† shows the nitrogen adsorption/desorption isotherms of RSSC-800 and SSC-X carbon materials, whereas Fig. S2b† shows the corresponding pore size distribution determined from the adsorption branch using DFT. Compared with the carbon samples derived from Sterculia scaphigera without adsorbing water, both the specific surface area and pore size distribution of the SSC-X specimens are quite distinctive. The values of porosity characteristics for all samples are provided in Table S1.† The specific area of SSC-700 carbon is as high as 481.74 m2 g−1; nevertheless, the RSSC-800 carbon sample only possesses a specific surface area of 2.19 m2 g−1. Simultaneously, the microporosity and microporous surface area increase effectively for the SSC-X specimens. These results synergistically suggest that water inhalation has a considerable improvement on the pore texture of the as-prepared carbon materials.
HRTEM was performed on RSSC-800 and SSC-800 carbon sample to peep at its features in the nanometer region, as displayed in Fig. 4d and e. No graphene sheets are found stacking along with the 002 plane of carbon, as shown in Fig. 4d. Interestingly, HRTEM image in Fig. 4e indicates the graphitized structure of the obtained SSC-800 carbon material, even though the graphite degree is lower than that of natural graphite. Furthermore, line profile, as shown in the inset of Fig. 4d, quantifies the d-spacing of several graphene sheets in the SSC-800 carbon, which can be generally assigned to the 002 plane of graphitic carbon and is in the scope of 0.347–0.424 nm. The graphite crystalline forming the expansion in the d-spacing of graphene layers may be in favour of accommodating extra lithium ions.25 The micropore textures caused by the stacking of turbostratic disorder structures, as shown in Fig. 4e, also offer additional sites to store more lithium on the pore surfaces.13,26
3.2 Electrochemical performance of SSC-X and RSSC-800 specimens
EIS measurement was initially employed to investigate coin cell assembled with carbonized samples. As illustrated in Fig. 5a, the EIS consists of a depressed semicircle at the range from high-frequency to middle-frequency and a slopping line at the low frequency region. The intersection of the real axis at the high frequency generally refers to the electronic and ionic resistance of the two electrodes and electrolyte/separator (Rel). According to the simulated equivalent circuit, as shown in Fig. S3,† the depressed semicircle is actually composed of two overlapping parts. The resistance in the high frequency is attributed to SEI film (Rf) and constant phase element (CPE) formed on the surface of two electrodes.27–29 The medium frequency one represents charge-transfer resistance (Rct) and the related double layer capacitance. For the latter, CPE is also introduced to precisely simulate the capacitance originated from the surface of active material. The following slope line at low frequency reflects the Warburg impedance of lithium ion diffusion.29 For the series of SSC-X samples, the resistance trend in the high and middle frequencies, which is roughly equal to the diameter of the semicircle in the spectra, indicates that lower carbonization temperature gives higher sum of Rf and Rct. Noticeably, the sample RSSC-800 shows the maximum Rel and relatively small semicircle. In principle, the variation of graphitization degree, specific surface area and pore structures are primarily responsible for the electrochemical discrepancy of these carbon electrodes. Further electrochemical characterization was performed at a current density of 0.1 C to distinguish specific capacity and cyclability of the three carbon electrodes, as shown in Fig. 5b. An evident feature of Fig. 5b is the excellent cyclic performance and reversibility within 100 cycles, although all of these carbon electrodes show large irreversible capacity in the initial cycle. Hence, these electrochemical behaviours are basically similar to soft carbon materials reported in the literature.14,19,30–32 Moreover, it is evident that, as shown in the inset of Fig. 5b, the SSC-800 electrode exhibits a specific capacity up to 423 mA h g−1 after 100 cycles and 97% coulombic efficiency, which is comparatively superior to the samples of SSC-700 (364 mA h g−1), SSC-900 (320 mA h g−1) and RSSC-800 (199 mA h g−1).CV curves of RSSC-800 and SSC-800 are depicted in Fig. 6a and b at a scan rate of 0.1 mV s−1 between 0.05 and 3.0 V. In the first discharge cycle, two distinct reduction peaks around 0.7 and 1.4 V, which are attributed to the irreversible reactions between carbon electrode and the co-intercalation of solvated lithium ions into graphitized carbon sheets,33–35 are observed for both samples. Another characteristic in Fig. 6a and b is the almost overlapping CV curves in the subsequent cycles, which to some extent confirms the stable and superior reversibility. However, the anodic peak at the potential range between 0 and 0.1 V, which is characteristic of graphitic carbon material, is nearly negligible for SSC-800 in comparison with sample RSSC-800. It suggests that the electrochemical behaviours are highly dependent on the physical textures. The enlarged specific surface area and developed novel micropores and mesopores from water assisted carbonization of Sterculia scaphigera are favourable for electrochemical activity enhancement. Fig. 6c shows the initial galvanostatic charge/discharge profiles of the RSSC-800 and SSC-800 electrode in the range of 0.05 to 3 V. The specific capacity calculated from the first discharge curve reaches up to 1539 mA h g−1 for SSC-800 electrode, whereas the specific capacity of RSSC-800 is only 885 mA h g−1. The corresponding charge capacity of the SSC-800 and RSSC-800 samples is 596 and 289 mA h g−1, respectively. Both electrodes show large irreversible capacity in the initial cycle. The major reasons responsible for that are the formation of SEI film and the decomposition of electrolytes22 at the voltage plateau of 0.9 V for SSC-800 electrode and 0.7 V for RSSC-800 electrode. The rate performance is shown in Fig. 6d. When the current density gradually increased from 0.05 to 0.1, 0.2, 0.5, 1, and 2 C (1 C = 372 mA g−1), the corresponding average discharge capacity decreased from 474 to 400, 340, 290, 253, and 130 mA h g−1, respectively. If the current density goes back to 0.05 and 0.1 C again, then the average discharge capacity returns to approximately 478 and 402 mA h g−1, respectively. These results indicate good rate capability and a very stable cycling performance. It is worth noting that despite the fact that the specific capacity of SSC-800 at a rate of 2 C is around 130 mA h g−1, the rate behaviour is superior to the pure graphite electrode32 and is also similar to 30 wt% soft carbon modified hard carbon anodic materials.36 In addition, although the RSSC-800 sample demonstrated even better rate performance, the reversible discharge capacity of SSC-800 sample is considerably higher than that of the RSSC-800 sample at the equal current density. The aforementioned results clearly demonstrate that the electrochemical performance of the prepared carbon material is significantly enhanced after water absorption. More possibilities to explain the enhanced electrochemical performance of the SSC-800 electrode is ascribed to the nanoscopic pore structure and graphitized degree synergistically offering a particular route and electric conductivity that enables rapid lithium ion diffusion. Consequently, the prepared carbon sample SSC-800 may be more suitable to serve as anode material for application in LIB.
4. Conclusions
Sterculia scaphigera was utilized for the first time as a novel carbonaceous precursor to prepare graphitized porous carbon materials. Sterculia scaphigera exhibits tremendous capability to adsorb a large amount of water, causing significant volume swelling. On this account, graphitized carbon with favourable micropore architecture can be prepared from the carbonization of Sterculia scaphigera at high temperature. It was found that the adsorbed water plays a critical role in promoting the graphitization and the formation of highly porous structure. As a result, the obtained carbon materials possessed superior electrochemical performance with high reversible capacity, moderate rate capability and extremely stable cycling properties. Two possible reasons may account for this performance enhancement. On one hand, the carbonized materials have developed abundant micropore structure because water is inhaled into the body of Sterculia scaphigera and evaporated subsequently. On the other hand, the graphitization degree of carbonized Sterculia scaphigera is effectively enhanced due to the formation of thin film analogues after water adsorption and evaporation. Consequently, the feasible, cost-effective and eco-friendly synthesis route presented in this study to obtain carbon material brings novel perspectives for fabricating high performance electrode materials for LIB from naturally available biomass.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21001117), the Starting-Up Funds of South University of Science and Technology of China (SUSTC) through the talent plan of the Shenzhen Government and the Shenzhen Peacock Plan (KQCX20140522150815065). S.Y. thanks the support from the Guangdong and Shenzhen Innovative Research Team Program (no. 2011D052, KYPT20121228160843692).Notes and references
- M. Pasta, C. D. Wessells, R. A. Huggins and Y. Cui, Nat. Commun., 2012, 3, 1149–1155 CrossRef PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- N. S. Choi, Y. Yao, Y. Cui and J. Cho, J. Mater. Chem., 2011, 21, 9825–9840 RSC.
- J. Chang, X. Huang, G. Zhou, S. Cui, P. B. Hallac, J. Jiang, P. T. Hurley and J. Chen, Adv. Mater., 2014, 26, 758–764 CrossRef CAS PubMed.
- Z. Zhu, S. Wang, J. Du, Q. Jin, T. Zhang, F. Cheng and J. Chen, Nano Lett., 2013, 14, 153–157 CrossRef PubMed.
- L. Hu, P. Zhang, H. Zhong, X. Zheng, N. Yan and Q. Chen, Chem.–Eur. J., 2012, 18, 15049–15056 CrossRef CAS PubMed.
- L. Zhou, H. Xu, H. Zhang, J. Yang, S. B. Hartono, K. Qian, J. Zou and C. Yu, Chem. Commun., 2013, 49, 8695–8697 RSC.
- L. W. Ji, Z. Lin, M. Alcoutlabi and X. W. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- A. Vu, Y. Qian and A. Stein, Adv. Energy Mater., 2012, 2, 1056–1085 CrossRef CAS PubMed.
- H. Nishihara and T. Kyotani, Adv. Mater., 2012, 24, 4473–4498 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novak, Adv. Mater., 1998, 10, 725–763 CrossRef CAS.
- J. Dahn, T. Zheng, Y. Liu and J. Xue, Science, 1995, 270, 590–593 CAS.
- F. Bonino, S. Brutti, M. Piana, S. Natale, B. Scrosati, L. Gherghel and K. Müllen, Electrochim. Acta, 2006, 51, 3407–3412 CrossRef CAS PubMed.
- M. Schroeder, M. Winter, S. Passerini and A. Balducci, J. Power Sources, 2013, 238, 388–394 CrossRef CAS PubMed.
- J. E. Chae, K. Annaka, K. Hong, S. I. Lee, H. Munakata, S. S. Kim and K. Kanamura, Electrochim. Acta, 2014, 130, 60–65 CrossRef CAS PubMed.
- H. Azuma, H. Imoto, S. I. Yamada and K. Sekai, J. Power Sources, 1999, 81, 1–7 CrossRef.
- Y. N. Jo, E. Y. Lee, H. Y. Jeong, Z. H. Lee, K. J. Hong, S. I. Lee and Y. J. Kim, Electrochemical Characteristics of Structure-modified Soft Carbon as an anode in Lithium Ion Batteries, ECS Meeting Abstracts, The Electrochemical Society, 2012, 10, 954 Search PubMed.
- F. Bonino, S. Brutti, P. Reale, B. Scrosati, L. Gherghel, J. Wu and K. MüLLEN, Adv. Mater., 2005, 17, 743–746 CrossRef CAS PubMed.
- M. Inagaki, New carbons-control of structure and functions, Elsevier, 2000 Search PubMed.
- J. Gong, H. Wu and Q. Yang, Carbon, 1999, 37, 1409–1416 CrossRef CAS.
- F. Zheng, Y. Yang and Q. Chen, Nat. Commun., 2014, 5, 1–10 Search PubMed.
- P. Trucano and R. Chen, Nature, 1975, 258, 136–137 CrossRef CAS PubMed.
- A. Souza Filho, A. Jorio, A. K. Swan, M. Ünlü, B. Goldberg, R. Saito, J. Hafner, C. Lieber, M. Pimenta and G. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 085417 CrossRef.
- E. Yoo, J. Kim, E. Hosono, H. S. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef CAS PubMed.
- B. O. Jeong, S. H. Jeong, M. S. Park, S. Kim and Y. Jung, J. Nanosci. Nanotechnol., 2014, 14, 7788–7792 CrossRef CAS PubMed.
- J. Zheng, M. Gu, H. Chen, P. Meduri, M. H. Engelhard, J. G. Zhang, J. Liu and J. Xiao, J. Mater. Chem. A, 2013, 1, 8464–8470 CAS.
- F. Y. Su, C. You, Y. B. He, W. Lv, W. Cui, F. Jin, B. Li, Q. H. Yang and F. Kang, J. Mater. Chem., 2010, 20, 9644–9650 RSC.
- V. Freger, Electrochem. Commun., 2005, 7, 957–961 CrossRef CAS PubMed.
- H. Higuchi, K. Uenae and A. Kawakami, J. Power Sources, 1997, 68, 212–215 CrossRef CAS.
- T. Iijima, K. Suzuki and Y. Matsuda, Synth. Met., 1995, 73, 9–20 CrossRef CAS.
- S. H. Jeong, J. Y. Koh, T. J. Kim and Y. Jung, Bull. Korean Chem. Soc., 2014, 35, 2357 CrossRef CAS.
- Z. J. Fan, J. Yan, T. Wei, G. Q. Ning, L. J. Zhi, J. C. Liu, D. X. Cao, G. L. Wang and F. Wei, ACS Nano, 2011, 5, 2787–2794 CrossRef CAS PubMed.
- Y. K. Choi, K. I. Chung, W. S. Kim and Y. E. Sung, Microchem. J., 2001, 68, 61–70 CrossRef CAS.
- Y. Chen, Z. Lu, L. Zhou, Y. W. Mai and H. Huang, Energy Environ. Sci., 2012, 5, 7898–7902 CAS.
- J. Wang, J. L. Liu, Y. G. Wang, C. X. Wang and Y. Y. Xia, Electrochim. Acta, 2012, 74, 1–7 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06215b |
| This journal is © The Royal Society of Chemistry 2015 |