Bridged-cyclodextrin supramolecular hydrogels: host–guest interaction between a cyclodextrin dimer and adamantyl substituted poly(acrylate)s†
Abstract
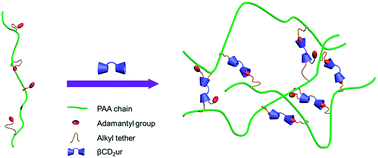
The formation of a hydrogel constructed from a cyclodextrin dimer (βCD2ur) and adamantyl substituted poly(acrylate) was investigated by 2D NOESY 1H NMR and isothermal titration calorimetry (ITC). The two methods generally demonstrate enhancement of complexation as the length of the hydrophobic tether increases, which is likely to be due to the cooperative effect. An optimal tether length of 6 was found to maximize the complexation between βCD2ur and the polymer. Increasing the tether length to twelve carbons slightly reduces the complexation affinity revealed by ITC experiments. Meanwhile, rheological analysis indicates the maximum degree of crosslinking was achieved by PAAADhn/βCD2ur complexation since the rigid structure of PAAAD restricts the crosslinking based on the host–guest complexation between the cyclodextrin dimer and adamantyl substituent and the competitive binding from the long alkyl tether in PAAADddn reduces the degree of crosslinking.

 Please wait while we load your content...
Please wait while we load your content...