Sonochemical synthesis of mesoporous ZrFe2O5 and its application for degradation of recalcitrant pollutants†
Abstract
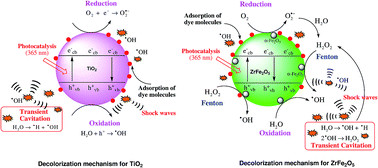
In this paper, we have reported the sonochemical synthesis and characterization of zirconium ferrite (ZrFe2O5), and its use as a catalyst in advanced oxidation processes (AOPs) using decolorization/degradation of azo and non-azo dyes as model processes. The efficacy of ZrFe2O5 for dye decolorization has been compared with the conventional catalyst TiO2 synthesized using sonochemical sol–gel method. Sonochemically synthesized ZrFe2O5 and TiO2 have been characterized using PSD, XRD, FESEM, TEM, DRS, BET and TGA. ZrFe2O5 is revealed to have a lower band gap energy than TiO2. However, the α-Fe2O3 (hematite) phase in ZrFe2O5 acts as a recombination center of photogenerated electrons and holes, which adversely affects the photo-activity of ZrFe2O5. In the context of degradation of recalcitrant pollutants, this adverse effect is compensated by Fenton activity of the α-Fe2O3 (hematite) phase which is stimulated by addition of H2O2. Sonochemically synthesized ZrFe2O5 also has high adsorption capacity for the textile dyes that assists their effective degradation through Fenton and photocatalytic mechanisms. The decolorization experiments have employed individual AOPs of sonolysis, photocatalysis, (heterogeneous) Fenton and various combinations of these as hybrid AOPs. Due to combined facets of photo- and Fenton-activity, ZrFe2O5 is a better catalyst for hybrid AOPs than TiO2. Nonetheless, the Fenton-activity of ZrFe2O5 overwhelms the photo-activity in dye decolorization. The most efficient hybrid AOP is revealed to be sono-photo-Fenton, in which both photo- and Fenton- activities of ZrFe2O5 are utilized. Analysis of decolorization experiments has also provided interesting mechanistic insight into interactions and synergies between different competing mechanisms of hybrid AOPs.


 Please wait while we load your content...
Please wait while we load your content...