Design, synthesis and biological evaluation of esculetin derivatives as anti-tumour agents†
Ping Wang‡
,
Yang-Liu Xia‡,
Yang Yu,
Jun-Xia Lu,
Li-Wei Zou,
Lei Feng,
Guang-Bo Ge* and
Ling Yang*
Laboratory of Pharmaceutical Resource Discovery, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: geguangbo@dicp.ac.cn; ylingdicp@gmail.com
First published on 2nd June 2015
Abstract
Esculetin, a naturally catecholic coumarin, possess multiple pharmacological activities including anti-tumour, anti-inflammatory and anti-oxidant. However, the extensive phase II metabolism and rapid elimination from the human body significantly hinder esculetin and its derivatives as drug leads/candidates. To improve both the metabolic stability and the anti-tumour activity of esculetin via rational modification, a series of C-4 and C-8 substituted derivatives were designed and synthesized by perchloric acid catalysed von Pechmann reaction and Mannich reaction, respectively. The in vitro metabolic half-life in human liver S9 and anti-tumour activities in A549 and B16 cell lines of the newly synthesized compounds were assayed. Of these compounds, 8-(pyrrolidin-1-ylmethyl)-4-trifluoromethyl esculetin 15 was the most potent candidate compound, with almost a 20-fold increase in antiproliferative activity and a 3-fold prolonged half-life in human liver S9 compared with the parent compound 1. In addition, the potential structure–activity relationship and structure–metabolic stability relationship were discussed. Notably, the introduction of a nitrogen containing group as a hydrogen bond acceptor at the C-8 position of esculetin can improve both the metabolic stability and anti-tumour activity. All of these findings are very helpful for the structural modification of esculetin and other bioactive phenolic compounds to improve their phase II metabolic stability and bioactivity synchronously.
Introduction
Coumarins are a large class of natural and synthetic compounds that have drawn much attention due to their remarkable biological activities, and several good druglikeness properties including excellent safety, high solubility and good permeability.1–5 Currently, several coumarins including warfarin,6 daphnetin7 and armillarisin A8 are clinically used drugs. Studies found that the existence of phenolic groups and their position on the coumarin skeleton are very important for its anti-tumour activities.9,10 The structure–activity relationships of various phenolic coumarins have revealed that the catechol group is the key pharmacophore for anti-tumour activities, and catecholic coumarins showed better anti-tumor activity than monohydroxy coumarins in several human tumour cell lines.11,12Esculetin (6,7-dihydroxycoumarin), a naturally occurring catecholic coumarin, widely distributed in many medicinal plants such as Artemisia capillaries, Citrus limonia and Euphorbia lathyris.13 Esculetin and its derivatives have been reported to inhibit the proliferation of a number of human malignant cell lines in vitro and have demonstrated activity against several types of animal tumours.14–17 Moreover, it also enhances taxol-induced apoptosis in human hepatocellular carcinoma cells (HepG2).18 In addition, esculetin has various bioactivities including lipoxygenase-inhibition,19 free radical scavenging,20 DNA protection21 and cancer chemoprevention.22 Hence, these cytotoxic coumarins represent an exploitable source of new anticancer agents, which might also help addressing side-toxicity and resistance phenomena. Although the pharmacological activities of esculetin and its analogs are recognized, the extensive phase II metabolism and rapid elimination from human body significantly hinder the development of these compounds as drug leads/candidates.23,24
From the view of chemical structure, the catechol group is not only the pharmacophore of coumarins but also the susceptible group for the attack of phase II metabolic enzymes. According to our previous study, the 7-OH of esculetin was the major site for UGT metabolism, whereas 6-OH could hardly be glucuronidated.25 In addition, it has been presented earlier that coumarin Mannich bases play a significant role as biologically active compounds in various diseases.26,27 Therefore, taking into account of previous data, to improve both the metabolic stability and the anti-tumour activities, in the present paper we devoted our efforts to design and synthesize some 4-substituted esculetins and 8-substituted esculetin Mannich bases as possible anti-tumour agents examining their ability to inhibit proliferation and their metabolic half-life in vitro. Furthermore, the preliminary conclusions regarding the structure–activity relationship of the metabolic stability and the anti-tumour properties of esculetin derivatives were discussed, which can provide helpful guidance for the structural modification of 6,7-dihydroxycoumarins and other bioactive phenolic compounds.
Results and discussion
Chemistry
A sulphuric acid or perchloric acid catalysed von Pechmann reaction is a common method to prepare 4-substituted coumarins in a laboratory scale.28,29 Knoevenagel, Wittig and Perkin reactions have also been used according to the different starting materials.30–32 However, we used a perchloric acid catalysed von Pechmann reaction at room temperature, this was expected to diminish side-reactions but may also have an effect on reaction time. Moreover, the perchloric acid is practical to use because it does not give rise to warming in contrast to sulphuric acid, which heats up in pouring the reaction mixture into the water. The desired 4-substituted esculetin derivatives were prepared as depicted in Scheme 1. The compound 2–5 were prepared via the condensation reaction of 1,2,4-phenenyl triacetate with properly substituted β-ketoesters in the presence of perchloric acid with the good yield. Subsequently, compound 6 was obtained by refluxing chloride 5 in the mixture of DMF and water with the yield of 83%. | ||
Scheme 1 Synthesis of compounds 2–6. Reagents and conditions: (a) HClO4, rt, 6–8 h; (b) DMF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), reflux, 20 h. 3), reflux, 20 h. | ||
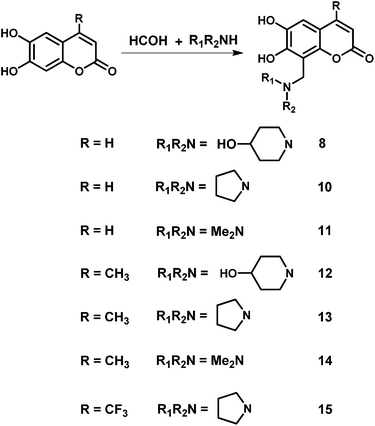
The structure of another series of derivatives combines the biological active group of 6,7-dihydroxycoumarin and the amine moiety as a new template. The synthesis of the coumarin derivatives 8–15 was accomplished according to the Mannich reaction as depicted in Scheme 2. Examples of hydroxycoumarin condensed with formaldehyde and amines have been reported previously.26,27,33,34 The synthesis of compound 13 was given in a previous publication.35 We have included them in our study in order to explore the potential structure–activity relationships, within these Mannich bases. In our case, esculetin/4-substituted esculetin, formaldehyde and the corresponding secondary amine in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
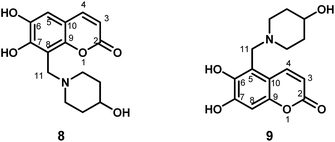
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 molar ratio were stirred in absolute ethanol or methanol at 25–60 °C for 8–12 h to yield the desired Mannich bases derivatives. The reactions were monitored by thin-layer chromatography. Esculetin condensed with formaldehyde and amines to compound 8–11 with the low yield and the poor regioselectivity. For example, esculetin reacted with formaldehyde and 4-hydroxy piperidine to yield compound 8 and compound 9 with the yield of 12% and 3%, respectively. The structure of compound 8 and 9 (Fig. 1) was confirmed by 1H NMR, 13C NMR, HSQC, HMBC and mass spectral analysis. Especially, the position of the aminomethyl group at coumarin was unambiguously corroborated by HMBC experiment. The HMBC spectrum of compound 8 showed two protons of C-11 (δ 4.00 ppm) correlated with C-7 (δ 146.8 ppm), C-8 (δ 106.8 ppm) and C-9 (δ 153.6 ppm), respectively. Meanwhile, the HMBC spectrum of compound 9 showed two protons of C-11 (δ 4.44 ppm) correlated with C-5 (δ 110.7 ppm), C-6 (δ 143.5 ppm) and C-10 (δ 112.9 ppm), respectively. Moreover, structural modifications at the C-4 position strongly affect the bioactivities of esculetins, so 4-substituted-esculetins were used as raw material to prepare a series of 8-substituted esculetin Mannich derivatives. Notably, 4-substituted esculetin condensed with formaldehyde and amines to compound 12–15 with the good yield and the excellent regioselectivity.
1.3 molar ratio were stirred in absolute ethanol or methanol at 25–60 °C for 8–12 h to yield the desired Mannich bases derivatives. The reactions were monitored by thin-layer chromatography. Esculetin condensed with formaldehyde and amines to compound 8–11 with the low yield and the poor regioselectivity. For example, esculetin reacted with formaldehyde and 4-hydroxy piperidine to yield compound 8 and compound 9 with the yield of 12% and 3%, respectively. The structure of compound 8 and 9 (Fig. 1) was confirmed by 1H NMR, 13C NMR, HSQC, HMBC and mass spectral analysis. Especially, the position of the aminomethyl group at coumarin was unambiguously corroborated by HMBC experiment. The HMBC spectrum of compound 8 showed two protons of C-11 (δ 4.00 ppm) correlated with C-7 (δ 146.8 ppm), C-8 (δ 106.8 ppm) and C-9 (δ 153.6 ppm), respectively. Meanwhile, the HMBC spectrum of compound 9 showed two protons of C-11 (δ 4.44 ppm) correlated with C-5 (δ 110.7 ppm), C-6 (δ 143.5 ppm) and C-10 (δ 112.9 ppm), respectively. Moreover, structural modifications at the C-4 position strongly affect the bioactivities of esculetins, so 4-substituted-esculetins were used as raw material to prepare a series of 8-substituted esculetin Mannich derivatives. Notably, 4-substituted esculetin condensed with formaldehyde and amines to compound 12–15 with the good yield and the excellent regioselectivity.
Metabolic stability assay in vitro
Metabolic stability refers to the susceptibility of compounds to biotransformation in the context of selecting and/or designing drugs with favourable pharmacokinetic properties. To identify the most promising drug candidates, thus reducing attrition at later stages and increasing success rates, the in vitro half-life (t1/2) of all the synthesized esculetin derivatives were evaluated in pooled human liver S9 fraction in the presence of the cofactors for glucuronidation, sulfation, and methylation, i.e., UDPGA, PAPS, and SAM. The results were shown in Table 1. Among seven 4-substituted esculetin derivatives, most of compounds demonstrated higher metabolic stability than the parent compound 1 with an exception of compound 3. Compound 7 was the most metabolically stable with elimination t1/2 value of 91.4 min, double longer compared with the parent compound 1. In contrast, compound 3 with phenyl group at this position was rapidly eliminated, with an elimination t1/2 value of 20.8 min. A simple structure–metabolic stability relationship analysis showed that hydrophilic substitution in the C-4 position of esculetin is more favorable to the improvement of metabolic stability. All of these 8-substituted esculetin Mannich derivatives demonstrated much higher metabolic stability than the parent compound 1, with elimination t1/2 values from 102 to 176 min. Among these analogs, compound 14 with a dimethylamino group demonstrated the most metabolically stable with a 3-fold longer t1/2 compared with the parent compound 1. From the view of structural features of these esculetin Mannich bases, the introduction of dimethylamino group to the C-8 site of esculetin demonstrated to be more effective strategy to enhance metabolic stability than the introduction of pyrrolidinyl group and piperidinol group to this position. It's possible due to that the intramolecular hydrogen bond energy of dimethylamino derivative is stronger than pyrrolidinyl derivative and piperidinol derivative. Moreover, it's worth mentioning that compound 9 exhibited a relatively short t1/2 compared with the other aminomethyl substituted esculetin derivatives, although there may be a intramolecular hydrogen bond formed between the aminomethyl group and 6-OH of esculetin. This confirmed our previous observation that the 7-OH of esculetin was the major site for UGT metabolism. On the other hand, the amine containing substitutes at C-8 position partially increased the solubility of these esculetin Mannich derivatives, which may contribute to the reduction of the substrate affinity to metabolic enzyme. Also the increased molecular volume could cause certain steric hindrance for substrate to enter the active site of enzyme. Taken together, all of these findings revealed that aminomethyl group introduced to the C-8 position, of which the amine group could interact with 7-OH of esculetin and form an intramolecular hydrogen bond, might play a significant role in improving metabolic stability. This strategy could be utilized to guide the structure modification towards improving phase II metabolic stability of 6,7-dihydroxycoumarins.| Compd | B16 IC50 (μM) | A549 IC50 (μM) | t1/2 in HS9 (min) |
|---|---|---|---|
| 1 | 71.4 ± 8.3 | 48.9 ± 10.3 | 44.7 |
| 2 | 29.2 ± 3.6 | 17.2 ± 3.1 | 57.8 |
| 3 | 16.8 ± 0.1 | 9.6 ± 2.1 | 20.8 |
| 4 | 13.2 ± 0.2 | 9.2 ± 1.8 | 49.1 |
| 5 | 18.4 ± 1.3 | 24.4 ± 2.2 | 47.4 |
| 6 | 52.4 ± 1.4 | >100 | 67.4 |
| 7 | 26.7 ± 0.1 | >100 | 91.4 |
| 8 | 15.3 ± 1.3 | 9.1 ± 0.1 | 102.4 |
| 9 | 16.5 ± 0.1 | 6.5 ± 0.3 | 96.9 |
| 10 | 9.3 ± 2.5 | 6.3 ± 0.4 | 124.9 |
| 11 | 28.7 ± 1.3 | 14.1 ± 0.8 | 140.9 |
| 12 | 12.1 ± 1.6 | 10.9 ± 2.3 | 118.1 |
| 13 | 5.5 ± 2.1 | 5.9 ± 0.4 | 165.3 |
| 14 | 16.6 ± 12.5 | 11.0 ± 3.4 | 176.4 |
| 15 | 3.8 ± 1.2 | 1.9 ± 0.5 | 115.3 |
Antiproliferative activity assays in vitro
Actually, in vitro cytotoxicity assays with cultured cells are widely used to chemicals including cancer chemotherapeutics, pharmaceuticals, biomaterials, natural toxins, antimicrobial agents and industrial chemicals because they are rapid and economical. To evaluate the anti-proliferative activity of all the synthesized compounds, the IC50 values for the inhibition on human cancer cell lines A549 (non-small cell lung carcinoma) and B16 (mouse melanoma cell) were tested using SRB (Sulforhodamine B) assay with esculetin 1 as the reference compound. The results shown in Table 1 demonstrated that most of the synthesized compounds displayed better anti-proliferative activity than the parent compound 1 with IC50 values in micromole range. Among seven 4-substituted esculetin derivatives, compound 4 gave the best results with 13.2 μM of IC50 value for B16 cell line and 9.6 μM for A549. Compound 2, 3 and 5, which possess CH3, Ph and CH2Cl in this position showed moderate activities. Whereas compounds 6 and 7 with CH2OH and CH2COOH in this position exhibited relatively poor activities, especially for A549 cell lines, and both of them with IC50 values over 100 μM. A simple structure–activity relationship analysis showed that the anti-proliferative activity closely related to the lipophilic substituted groups at C-4 position, which showed a contrasting effect on the metabolic stability. Among 8-substituted aminomethyl esculetin derivatives, the anti-proliferative activity of most of the synthesized compounds (with 28.7 μM to 3.8 μM of IC50 values for B16, and 14.1 μM to 1.9 μM for A549) significantly increased compared with the parent compound 1 (IC50 71.4 μM and 48.9 μM for B16 and A549, respectively). Concerning the structural features of the esculetin Mannich bases, esculetin derivatives with a pyrrolidinyl group in C-8 position of esculetin exhibited higher anti-proliferative activity than the derivatives with a dimethylamino or piperidinol group. Subsequently, it's worth mentioning that compound 15 containing both trifluoromethyl and pyrrolidinyl group was designed, and an almost 20-fold increase in anti-proliferative activity was observed. Taken together, it seems that the aminomethyl moiety of these compounds might play a significant role, meanwhile the lipophilic substituents at C-4 position slightly enhanced the anti-proliferative activity.Conclusion
In summary, to improve both the metabolic stability and the anti-tumour activity of esculetins, a series of 4-substituted esculetins and 8-substituted esculetin Mannich bases were designed and synthesized by perchloric acid catalysed von Pechmann reaction and Mannich reaction. All the newly synthesized compounds were evaluated for their metabolic half-life in pooled human liver S9 fraction and in vivo anti-tumour activity in B16 and A549 cell lines. The assay results showed that most of the synthesized compounds displayed better anti-proliferative activity than the parent compound 1 with IC50 values in micromole range. Concerning the structural features, aminomethyl group introduced to C-8 position, of which the amine group could interact with 7-OH of esculetin and form an intramolecular hydrogen bond, might play a significant role for both the metabolic stability and the anti-tumour activity, while the lipophilic substitution in C-4 position led to a slightly enhanced anti-proliferative activity and hydrophilic substitution in C-4 position was more favourable to the improvement of metabolic stability. These findings could provide useful guidance for the structure modification towards improving phase II metabolic stability.Experimental
Materials and methods
Compound 1 (esculetin) and compound 7 (4-acetic acid esculetin) was purchased from the Boyle company (shanghai, China). Alamethicin, Brij 58, magnesium chloride, D-saccharic acid, 1,4-lactone, β-glucuronidase (EC no. 3.2.1.31), uridine 5′-diphospho-glucuronic acid trisodium salt (UDPGA), 3′-phosphoade-nosin-5′-phosphosulfate (PAPS), S-adenosyl-L-methionine (SAM) and niflumic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pooled human liver S9 fraction (HS9) was purchased from Research Institute for Liver Diseases (Shanghai, China). sulforhodamine B (SRB) were purchased from Sigma-Aldrich (St. Louis, MO, USA), A549 and B16 were purchased from the Committee on Type Culture Collection of Chinese Academy of Sciences (Shanghai, China).All reagents used in the synthesis were obtained commercially and used without further purification. The 1H NMR and 13C NMR spectra were recorded in DMSO-d6 using a Bruker ARX 400 spectrometer (400 MHz for 1H NMR and 100 MHz for 13C NMR), and chemical shifts were expressed as ppm against TMS as an internal reference. The reactions were monitored by thin layer chromatography (TLC) on glass-packed precoated silica gel GF254 plates and visualized in an iodine chamber or with a UV lamp. Flash column chromatography was performed using silica gel (200–300 mesh) purchased from Qingdao Haiyang Chemical Co. Ltd. High-resolution mass spectral (HRMS) analyses were measured with Hybrid Ion Trap-Orbitrap Mass Spectrometer (LTQ Orbitrap XL, Thermo).
Esculetin and its derivatives were analyzed by a ultra-fast liquid chromatography spectrometry system (Shimadzu, Kyoto, Japan), equipped with two LC-20AD pumps, a DGU-20A3 vacuum degasser, a SIL-20ACHT auto-sampler, a CTO-20AC column oven, an SPD-M 20A diode-array detector (DAD), a CBM-20A communications bus module, a mass detector (2010 EV) with an electrospray ionization (ESI) interface, and a computer equipped with UFLC-MS Solution version 3.41 software. A Hedera C18 (150.0 mm × 2.1 mm, 3 μm) analytical column was used and column temperature was kept at 40 °C. The mobile phase was acetonitrile (A) and 0.2% formic acid water (B) at a flow rate of 0.4 mL min−1, with a gradient: 0–6.0 min, 95% B-65% B; 6.0–9.0 min, 5% B; 9.0–15 min, balance to 95% B.
General procedure for the synthesis of 4-substituted esculetins (2–5)
To a mixture of 1,2,4-phenenyl triacetate (5.0 mmol) and properly substituted β-ketoesters (10.0 mmol) was added drop-wise perchloric acid (5.0 mL) at room temperature and stirred for 6–8 h. After completion of the reaction as indicated by TLC, the reaction mixture was poured slowly into a mixture of ice-water (100 mL) with stirring. The resultant suspension was filtered and the collected solid was washed with water and dried, then crude compound was recrystallized from methanol to afford product.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.73 (s, 1H, ArH), 7.00 (s, 1H, ArH), 9.34 (s, br, 1H, ArOH), 10.19 (s, br, 1H, ArOH); 13C NMR (100 MHz, DMSO-d6) δ: 18.68, 103.13, 109.89, 110.86, 111.96, 143.24, 148.17, 150.58, 153.68, 161.08; ESI-MS: M = 192, found 191.0 [M − H]−.
C), 6.73 (s, 1H, ArH), 7.00 (s, 1H, ArH), 9.34 (s, br, 1H, ArOH), 10.19 (s, br, 1H, ArOH); 13C NMR (100 MHz, DMSO-d6) δ: 18.68, 103.13, 109.89, 110.86, 111.96, 143.24, 148.17, 150.58, 153.68, 161.08; ESI-MS: M = 192, found 191.0 [M − H]−.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.80 (s, 1H, ArH), 6.84 (s, 1H, ArH), 7.50–7.58 (m, 5H, ArH) 9.43 (s, br, 1H, ArOH), 10.26 (s, br, 1H, ArOH); ESI-MS: M = 254, found 253.0 [M − H]−.
C), 6.80 (s, 1H, ArH), 6.84 (s, 1H, ArH), 7.50–7.58 (m, 5H, ArH) 9.43 (s, br, 1H, ArOH), 10.26 (s, br, 1H, ArOH); ESI-MS: M = 254, found 253.0 [M − H]−.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.86 (s, 1H, ArH), 7.03 (s, 1H, ArH), 9.78 (s, br, 1H, ArOH), 10.61 (s, br, 1H, ArOH); 13C NMR (100 MHz, DMSO-d6) δ: 104.01, 105.05, 108.89, 112.12, 120.94, 123.68, 144.03, 149.59, 152.08, 159.62; ESI-MS: M = 246, found 245.1 [M − H]−.
C), 6.86 (s, 1H, ArH), 7.03 (s, 1H, ArH), 9.78 (s, br, 1H, ArOH), 10.61 (s, br, 1H, ArOH); 13C NMR (100 MHz, DMSO-d6) δ: 104.01, 105.05, 108.89, 112.12, 120.94, 123.68, 144.03, 149.59, 152.08, 159.62; ESI-MS: M = 246, found 245.1 [M − H]−.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.78 (s, 1H, ArH), 7.12 (s, 1H, ArH), 9.48 (s, br, 1H, ArOH), 10.39 (s, br, 1H, ArOH). ESI-MS: M = 225.5, found 224.9 [M − H]− and 226.9 [M − H]−.
C), 6.78 (s, 1H, ArH), 7.12 (s, 1H, ArH), 9.48 (s, br, 1H, ArOH), 10.39 (s, br, 1H, ArOH). ESI-MS: M = 225.5, found 224.9 [M − H]− and 226.9 [M − H]−.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.75 (s, 1H, ArH), 6.94 (s, 1H, ArH), 9.31 (s, br, 1H, ArOH), 10.19 (s, br, 1H, ArOH); ESI-MS: M = 208, found 208.9 [M + H]+.
C), 6.75 (s, 1H, ArH), 6.94 (s, 1H, ArH), 9.31 (s, br, 1H, ArOH), 10.19 (s, br, 1H, ArOH); ESI-MS: M = 208, found 208.9 [M + H]+.General procedure for the synthesis of 8-substituted esculetin Mannich bases (8–15)
To a solution of the appropriate amines (0.013 mol) in 30 mL of absolute methanol was added aqueous formaldehyde (37%) (0.015 mol). After 0.5 h of gentle refluxing at 50 °C, esculetin/4-substituted esculetin was added (0.01 mol), dissolved in 50 mL of absolute methanol. The duration of the reaction was 8–12 h and the reaction was monitored by TLC.Compound 8. 1H NMR (400 MHz, DMSO-d6) δ: 1.48 (m, 2H, CH2), 1.82 (m, 2H, CH2), 2.49 (m, 2H, NCH2), 2.91 (m, 2H, NCH2), 3.62 (m, 1H, CHOH), 4.00 (s, 2H, CH2), 6.08 (d, J = 8 Hz, 1H, COCH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.89 (s, 1H, ArH),7.81 (d, J = 8 Hz, 1H, PhCH
C), 6.89 (s, 1H, ArH),7.81 (d, J = 8 Hz, 1H, PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (100 MHz, DMSO-d6) δ: 33.50, 49.72, 52.56, 106.77, 108.87, 109.71, 110.38, 142.79, 144.87, 146.79, 153.60, 160.62; ESI-MS: M = 291, found 292.1 [M + H]+; HRMS (ESI): C15H17NO5 calcd for [M + H]+: 292.1185, found: 292.1181.
C); 13C NMR (100 MHz, DMSO-d6) δ: 33.50, 49.72, 52.56, 106.77, 108.87, 109.71, 110.38, 142.79, 144.87, 146.79, 153.60, 160.62; ESI-MS: M = 291, found 292.1 [M + H]+; HRMS (ESI): C15H17NO5 calcd for [M + H]+: 292.1185, found: 292.1181.
Compound 9. 1H NMR (400 MHz, DMSO-d6) δ: 1.68 (m, 2H, CH2), 1.92 (m, 2H, CH2), 3.09 (m, 2H, NCH2), 3.29 (m, 2H, NCH2), 3.76 (m, 1H, CHOH), 4.44 (s, 2H, CH2), 6.27 (d, J = 8 Hz, 1H, COCH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.97 (s, 1H, ArH), 8.33 (d, J = 8 Hz, 1H, PhCH
C), 6.97 (s, 1H, ArH), 8.33 (d, J = 8 Hz, 1H, PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (100 MHz, DMSO-d6) δ: 30.38, 48.53, 49.61, 103.75, 110.68, 111.56, 112.92, 141.95, 143.54, 148.89, 150.28, 160.18; ESI-MS: M = 291, found 292.1 [M + H]+; HRMS (ESI): C15H17NO5 calcd for [M + H]+: 292.1185, found: 292.1183.
C); 13C NMR (100 MHz, DMSO-d6) δ: 30.38, 48.53, 49.61, 103.75, 110.68, 111.56, 112.92, 141.95, 143.54, 148.89, 150.28, 160.18; ESI-MS: M = 291, found 292.1 [M + H]+; HRMS (ESI): C15H17NO5 calcd for [M + H]+: 292.1185, found: 292.1183.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.82 (s, 1H, ArH), 7.76 (d, J = 8.0 Hz, 1H, PhCH
C), 6.82 (s, 1H, ArH), 7.76 (d, J = 8.0 Hz, 1H, PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (100 MHz, DMSO-d6) δ: 23.05 (2C), 49.88, 52.84 (2C), 105.98, 107.16, 107.75, 109.05, 143.49, 144.90, 147.58, 156.57, 160.86; ESI-MS: M = 261, found 262.1 [M + H]+; HRMS (ESI): C14H15NO4 calcd for [M + H]+: 262.1079, found: 262.1072.
C); 13C NMR (100 MHz, DMSO-d6) δ: 23.05 (2C), 49.88, 52.84 (2C), 105.98, 107.16, 107.75, 109.05, 143.49, 144.90, 147.58, 156.57, 160.86; ESI-MS: M = 261, found 262.1 [M + H]+; HRMS (ESI): C14H15NO4 calcd for [M + H]+: 262.1079, found: 262.1072.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.86 (s, 1H, ArH), 7.79 (d, J = 12.0 Hz, 1H, PhCH
C), 6.86 (s, 1H, ArH), 7.79 (d, J = 12.0 Hz, 1H, PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 8.16 (s, 1H, ArOH); ESI-MS: M = 235, found 236.1 [M + H]+.
C), 8.16 (s, 1H, ArOH); ESI-MS: M = 235, found 236.1 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.94 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.30, 33.61, 49.77, 52.88, 107.02, 107.58, 109.18, 109.77, 142.49, 145.82, 152.80, 153.61, 160.32; ESI-MS: M = 305, found 306.1 [M + H]+; HRMS (ESI): C16H19NO5 calcd for [M + H]+: 306.1341, found: 306.1337.
C), 6.94 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.30, 33.61, 49.77, 52.88, 107.02, 107.58, 109.18, 109.77, 142.49, 145.82, 152.80, 153.61, 160.32; ESI-MS: M = 305, found 306.1 [M + H]+; HRMS (ESI): C16H19NO5 calcd for [M + H]+: 306.1341, found: 306.1337.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.89 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.89, 23.60, 50.17, 53.33, 106.97, 108.32, 108.70, 143.65, 146.90, 154.23, 155.87, 161.07; ESI-MS: M = 275, found 276.1 [M + H]+; HRMS (ESI): C15H17NO4 calcd for [M + H]+: 276.1236, found: 276.1230.
C), 6.89 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.89, 23.60, 50.17, 53.33, 106.97, 108.32, 108.70, 143.65, 146.90, 154.23, 155.87, 161.07; ESI-MS: M = 275, found 276.1 [M + H]+; HRMS (ESI): C15H17NO4 calcd for [M + H]+: 276.1236, found: 276.1230.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.92 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.87, 43.83, 54.51, 106.93, 107.48, 108.82, 109.27, 143.39, 146.82, 154.21, 155.03, 160.98; ESI-MS: M = 249, found 250.0 [M + H]+; HRMS (ESI): C13H15NO4 calcd for [M + H]+: 250.1079, found: 250.1074.
C), 6.92 (s, 1H, ArH); 13C NMR (100 MHz, DMSO-d6) δ: 18.87, 43.83, 54.51, 106.93, 107.48, 108.82, 109.27, 143.39, 146.82, 154.21, 155.03, 160.98; ESI-MS: M = 249, found 250.0 [M + H]+; HRMS (ESI): C13H15NO4 calcd for [M + H]+: 250.1079, found: 250.1074.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.71 (s, 1H, ArH); 13C NMR (100 MHz, d-DMSO) δ: 23.27, 49.55, 53.45, 98.26, 102.01, 102.07, 103.10, 104.17, 146.41, 151.66, 160.33, 164.84; ESI-MS: M = 329, found 330.1 [M + H]+; HRMS (ESI): C15H14F3NO4 calcd for [M + H]+: 330.0953, found: 330.0946.
C), 6.71 (s, 1H, ArH); 13C NMR (100 MHz, d-DMSO) δ: 23.27, 49.55, 53.45, 98.26, 102.01, 102.07, 103.10, 104.17, 146.41, 151.66, 160.33, 164.84; ESI-MS: M = 329, found 330.1 [M + H]+; HRMS (ESI): C15H14F3NO4 calcd for [M + H]+: 330.0953, found: 330.0946.Metabolic stability assay in vitro
To determine the effects of a combination of glucuronidation, sulfation, and methylation, the 6,7-dihydroxycoumarins were incubated with S9 fraction in the presence of three cofactors (2 mM UDPGA, 0.2 mM PAPS and 0.2 mM SAM). The incubation mixture contained pooled human S9 fraction (0.02 mg mL−1 protein), 5 mM MgCl2, 2 mM UDPGA, 0.2 mM PAPS, 0.2 mM SAM and 5 μM esculetin derivatives (dissolved in DMSO, final concentration 1%) in 200 μL of 50 mM Tris buffer (pH 7.4), as described in the reported manuscript.36 Controls were incubated in the absence of UDPGA, PAPS and SAM. After incubation at 37 °C for 0 to 40 min, the reactions were terminated by adding 200 μL of cold acetonitrile. The samples were centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 20 min. The supernatants (200 μL) were subjected to assay for the time-dependent esculetins depletion using HPLC.
000 g for 20 min. The supernatants (200 μL) were subjected to assay for the time-dependent esculetins depletion using HPLC.
Cell culture and maintenance
The human cancer cell lines A549 (non-small cell lung carcinoma) and B16 (mouse melanoma cell) were obtained from American Type Culture Collection (ATCC), which were cultivated in the Dulbecco's modified. Eagle medium (DMEM, GIBCO), containing 10% (v/v) heat inactivated fetal bovine serum (FBS, GIBCO), 100 IU mL−1 penicillin and 100 μg mL−1 streptomycin (GIBCO) in culture dish in a humidified atmosphere of 5% CO2 at 37 °C.Antiproliferative activity assays in vitro
The monolayer cell culture was trypsinized and the cell count was adjusted to 0.5–1.0 × 105 cells per mL using medium containing 10% new born sheep serum. To each well of the 96 well microtitre plate, 0.1 mL of the diluted cell suspension (approximately 7000 cells) was added. After 24 hours, when a partial monolayer was formed, the supernatant was flicked off, washed once and 100 μL of different test compound concentrations were added to the cells in microtitre plates. The plates were then incubated at 37 °C for 48 hours in 5% CO2 incubator and microscopic examination was carried out and observations recorded every 24 hours. After 48 hours, 100 μL of 10% trichloroacetic acid was added to the wells gently such that it forms a thin layer over the test compounds. The plates were incubated at 4 °C for one hour. The plates were flicked and washed five times with tap water to remove traces of medium, sample and serum, and were then air-dried. The air-dried plates were stained with 40 μL SRB and kept for 30 minutes at room temperature. The plates were then air-dried. 100 μL of 10 mM Tris base was then added to the wells to solubilize the dye. The plates were shaken vigorously for 5 minutes. The absorbance was measured using microplate reader at a wavelength of 540 nm. All experiments were repeated at least three times. The data were calculated using Graph Pad Prism version 6.0. The IC50 values were fitted using a nonlinear regression model with a sigmoidal dose response.Acknowledgements
This work was supported by the National S&T Major Projects of China (2012ZX09501001, 2012ZX09506001), the National Basic Research Program of China (2013CB531800), and NSF of China (81402822, 81273590, and 81302793).Notes and references
- F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS.
- L. Wu, X. Wang, W. Xu, F. Farzaneh and R. Xu, Curr. Med. Chem., 2009, 16, 4236–4260 CrossRef CAS.
- M. E. Riveiro, N. De Kimpe, A. Moglioni, R. Vazquez, F. Monczor, C. Shayo and C. Davio, Curr. Med. Chem., 2010, 17(13), 1325–1338 CrossRef CAS.
- M. J. Matos, L. Santana, E. Uriarte, G. Delogu, M. Corda, M. B. Fadda, B. Era and A. Fais, Bioorg. Med. Chem. Lett., 2011, 21, 3342 CrossRef CAS PubMed.
- A. Galkin, A. Fallarero and P. M. Vuorela, J. Pharm. Pharmacol., 2009, 61(2), 177–184 CrossRef CAS.
- P. Petersen, J. Godtfredsen, G. Boysen, E. Andersen and B. Andersen, Lancet, 1989, 333(8631), 175–179 CrossRef.
- Y. Z. Yang, A. Ranz, H. Z. Pan, Z. N. Zhang, X. B. Lin and S. R. Meshnick, Am. J. Trop. Med. Hyg., 1992, 46(1), 15–20 CAS.
- Y. Wang, P. Li, Y. Tang, J. P. Fawcett and J. Gu, J. Pharm. Biomed. Anal., 2007, 43(5), 1860–1863 CrossRef CAS PubMed.
- U. S. Weber, B. Steffen and C. P. Siegers, Res. Commun. Mol. Pathol. Pharmacol., 1998, 99(2), 193–206 CAS.
- M. A. Elasco-Velazquez, J. Agramonte-Hevia, D. Barrera, A. Jimenez-Orozco, M. J. Garcia-Mondragon, N. Mendoza-Patiño, A. Landa and J. Mandoki, Cancer Lett., 2003, 198, 179–186 CrossRef.
- I. Kostova, Anti-Cancer Agents Med. Chem., 2005, 5(1), 29–46 CrossRef CAS.
- H. Kolodziej, O. Kayser, H. J. Woerdenbag, W. van Uden and N. Pras, Z. Naturforsch., C: J. Biosci., 1997, 52, 240–244 CAS.
- Y. Masamoto, H. Ando, Y. Murata, Y. Shimoishi, M. Tada and K. Takahata, Biosci., Biotechnol., Biochem., 2003, 67, 631–634 CrossRef CAS PubMed.
- C. C. Chu, Y. Y. Tsai, C. J. Wang, W. L. Lin and T. H. Tseng, Eur. J. Pharmacol., 2001, 416, 25–32 CrossRef CAS.
- C. J. Wang, Y. J. Hsieh, C. Y. Chu, Y. L. Lin and T. H. Tseng, Cancer Lett., 2002, 183, 163–168 CrossRef CAS.
- S. H. Lee, C. Park, C. Y. Jin, G. Y. Kim, S. K. Moon, J. W. Hyun, W. H. Lee, B. T. Choi, T. K. Kwon, Y. H. Yoo and Y. H. Choi, Biomed. Pharmacother., 2008, 62, 723–729 CrossRef CAS PubMed.
- C. Park, C. Y. Jin, G. Y. Kim, I. W. Choi, K. K. Taeg, T. C. Byung, J. L. Su, H. L. Won and H. C. Yung, Toxicol. Appl. Pharmacol., 2008, 227, 219–228 CrossRef CAS PubMed.
- H. C. Kuo, H. J. Lee, C. C. Hu, H. I. Shun and T. H. Tseng, Toxicol. Appl. Pharmacol., 2006, 210, 55–62 CrossRef CAS PubMed.
- K. T. Lee and D. A. Lillard, J. Food Lipids, 1997, 4(2), 119–127 CrossRef CAS PubMed.
- M. Payá, B. Halliwell and J. R. Hoult, Biochem. Pharmacol., 1992, 44, 205–214 CrossRef.
- S. Tahara, N. Baba, M. Matsuo and T. Kaneko, Biosci., Biotechnol., Biochem., 2005, 69, 620–622 CrossRef CAS.
- K. Matsunaga, N. Yoshimi, Y. Yamada, M. Shimizu, K. Kawabata, Y. Ozawa, A. Hara and H. Mori, Jpn. J. Cancer Res., 1998, 89, 496–501 CrossRef CAS PubMed.
- S. C. Liang, G. B. Ge, H. X. Liu, Y. Y. Zhang, L. M. Wang, J. W. Zhang, L. Yin, W. Li, Z. Z. Fang and L. Yang, Drug Metab. Dispos., 2010, 38, 973–980 CrossRef CAS PubMed.
- Y. L. Xia, S. C. Liang, L. L. Zhu, G. B. Ge, G. Y. He, J. Ning, X. Lv, X. C. Ma, L. Yang and S. L. Yang, Drug Metab. Pharmacokinet., 2014, 29(2), 135–140 CrossRef CAS.
- Y. L. Xia, G. B. Ge, P. Wang, S. C. Liang, J. Ning, X. K. Qian, Y. Li and L. Yang, Drug Metab. Dispos., 2015, 43(4), 553–560 CrossRef CAS PubMed.
- R. B. Desai, J. Org. Chem., 1961, 26, 5251–5253 CrossRef CAS.
- C. A. Kontogiorgis and D. J. Hadjipavlou-Litina, J. Med. Chem., 2005, 48, 6401 CrossRef PubMed.
- M. Bulut and C. Erk, Dyes Pigm., 1996, 30, 99–104 CrossRef CAS.
- H. V. Pechmann, Ber. Dtsch. Chem. Ges., 1884, 17, 929–936 CrossRef PubMed.
- J. March, Advanced Organic chemistry, John Wiley & Sons, New York, 4th edn, 1992 Search PubMed.
- M. M. Garazd, Y. L. Garazd and V. P. Khilya, Chem. Nat. Compd., 2005, 41(3), 245–271 CrossRef CAS PubMed.
- J. M. Timonen, R. M. Nieminen and O. Sareila, Eur. J. Med. Chem., 2011, 46(9), 3845–3850 CrossRef CAS PubMed.
- V. N. Gupta, B. R. Sharima and R. B. Avora, J. Sci. Ind. Res., Sect. B, 1961, 20, 300 Search PubMed.
- M. G. Patel and S. Sethna, J. Indian Chem. Soc., 1962, 39, 595–598 CAS.
- J. Zak, D. Ron, E. Riva, H. P. Harding, B. C. Cross and I. R. Baxendale, Chem.–Eur. J., 2012, 18(32), 9901–9910 CrossRef CAS PubMed.
- X. Wen and T. Walle, Xenobiotica, 2006, 36, 387–397 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The 1H NMR, 13C NMR, HSQC, HMBC and HRMS spectra for synthesized compounds. See DOI: 10.1039/c5ra06070b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |