Fabrication of Cu–Ag bimetal nanotube-based copper silicates for enhancement of antibacterial activities†
Abstract
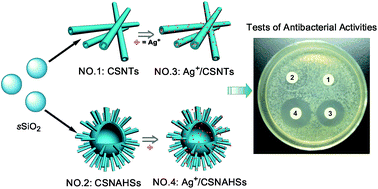
A novel Cu–Ag bimetal antibacterial system is reported. In this Cu–Ag bimetal system, copper silicate nanotubes (CSNTs) and copper silicate nanotube-assembled hollow nanospheres (CSNAHSs) with high BET surface area, unique hollow structure and strong antibacterial activity, were adopted as a carrier for loading silver ions to fabricate the copper–silver bimetal antibacterial agents (designated as Ag+/CSNTs and Ag+/CSNAHSs). Additionally, the antibacterial activity of Ag+/CSNTs and Ag+/CSNAHSs was investigated against Gram-negative bacteria (Escherichia coli BL21 and Escherichia coli JM109) and Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis). The results demonstrated that both silver-loaded copper silicates were highly effective against the four types of bacterial strains, and the Ag+/CSNAHSs showed a stronger antibacterial ability than Ag+/CSNTs due to their more silver loading contents. More importantly, a synergistic effect of copper ions and silver ions on the inhibition of the bacterial growth was observed in our bimetal antibacterial system.

 Please wait while we load your content...
Please wait while we load your content...