pH/temperature dependent selective removal of trace Cr(vi) from aqueous solution by imidazolium ionic liquid functionalized magnetic carbon nanotubes†
Abstract
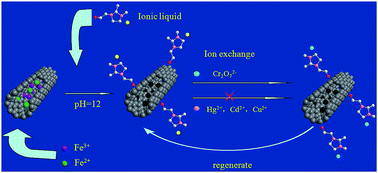
Carbon nanotubes have excellent adsorption property for metal ions. However, they lack selectivity and are difficult to separate from solutions. To resolve these problems, magnetic carbon nanotubes were prepared and functionalized with an imidazolium ionic liquid in this work. The functionalized magnetic carbon nanotube was used to remove Cr(VI) from water. It was found that the removal was highly selective and sensitive. At acidic conditions, 90% of Cr(VI) could be selectively removed on the ppb level with the coexistence of a high concentration of cations like Hg2+, Cd2+ and anions such as NO3− and SO42−. After the adsorption, the material could be collected easily by an external magnet, and then regenerated effectively by using 8% hydrazine hydrate. The high adsorption sensitivity, selectivity and capacity were attributed to the favorable electrostatic attraction, anion exchange affinity and entropy effects. Kinetic analysis indicated that the adsorption process of Cr2O72− was well described by a pseudo second order model. The adsorption isothermal analysis revealed that the adsorption process was endothermic, and could be described by a Langmuir model. In addition, it is interesting to find that unlike the commonly used absorbents, the adsorption capacity of the functionalized material for Cr2O72− increased with increasing temperature.

 Please wait while we load your content...
Please wait while we load your content...