Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media
M. Ajmalab,
M. Siddiqa,
N. Aktasc and
N. Sahiner*b
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad 45320, Pakistan
bCanakkale Onsekiz Mart University, Faculty of Science and Arts, Chemistry Department, Terzioglu Campus, 17020 Canakkale, Turkey. E-mail: sahiner71@gmail.com; Fax: +90 2862181948; Tel: +90 2862180010 ext. 2041
cChemical Engineering Departments, Yuzuncu Yil University, Campus, Van, 65080, Turkey
First published on 11th May 2015
Abstract
Poly(methacrylic-co-acrylonitrile) (p(MAc-co-AN)) microgels were prepared and nitrile groups were converted to amidoxime groups by chemical modification. Amidoximated microgels, amid-p(MAc-co-AN) microgels were used for in situ synthesis of cobalt–iron (Co–Fe) bimetallic magnetic nanoparticles by simultaneous reduction of Co(II) and Fe(II) ions within microgel. The prepared magnetic microgels as amid-mag-p(MAc-co-AN) microgel were found to be very effective adsorbents for the removal of metal ion such as Cd(II), Cr(III), and organic dyes e.g., methylene blue (MB), rhodamine 6G (R6G) and a herbicide, paraquat (PQ). A tremendous increase in the adsorption capacities of amid-p(MAc-co-AN) microgels was found as 88.1, 89.9, 190.0, 334.5 and 166.5 mg g−1 from 40.2, 37.4, 75.3, 57.4, and 56.3 for MB, R6G, PQ, Cd(II), and Cr(III), respectively. Moreover, a further increase in adsorption capacity of amid-mag-p(MAc-co-AN) microgel composites were also accomplished with the existence of magnetic particles. Adsorption of these contaminants from tap, river and seawater was also studied. The effects of different parameters i.e., pH, concentration of adsorbent solution and amount of adsorbate was also studied. Langmuir, Freundlich and Temkin adsorption models were applied, and the adsorption of Cd(II) and Cr(III) was found to obey Langmuir adsorption isotherm better.
Introduction
Water is the most essential element to sustain all living organisms. Unfortunately, it is being subjected to harmful contamination by various industrial operations, the use of different vehicles and by the waste of many industries based upon new technologies which have been developed in order to fulfil the demands of this modern era and generate wastes more than ever. Each source of contamination has its own destructive effects on plants, animals and ultimately on human health, but contamination of heavy metals in water is of serious concern due to their persistence in the environment and carcinogenicity to human beings.1 The heavy metals that include As, Co, Cu, Cd, Pb, Cr, Ni, Hg and Zn contaminate waters by many industries such as mining,2 tanneries,3 metal smelting,4 and batteries5 and so on. As these metals cannot be degraded biologically,6,7 their contamination in water becomes a potential threat for the entire ecosystem starting from microorganisms to human beings. In addition to heavy metals, organic dyes released from paper, plastic and cosmetic industries are also other source of contaminants for waters that are discharged from industrial wastes. The presence of these dyes in environment, specifically in water, can cause some severe effects on living organism and human health such as cyanosis, jaundice, shock, vomiting, quadriplegia, tissue necrosis and increase in heartbeat.8 Some dyes and their degradation products are so toxic that they are considered as carcinogenic.9 Another class of water pollutants is herbicides, which are mostly contaminated in water from irrigation system. Being a non-selective herbicide, paraquat is one the most commonly used herbicide throughout the world. Along with the advantage of quick action of paraquat to kill the unwanted plants, it has also destructive effects on human's health, because upon ingestion it causes pulmonary fibrosis and liver, heart and kidney failure as reported by Centre for Disease Control. Therefore, in order to preserve the entire ecosystem from sever damaging effects of heavy metals, organic dyes and herbicides, their removal from waters has become the subject of worldwide attention.10–12 Different techniques such as reverse osmosis, electrochemical treatments,13 precipitation, ion exchange, adsorption,14,15 membrane separation, evaporation, coagulation, flotation, hyper filtration,15,16 biosorption and oxidation processes17,18 have been introduced to remove toxic contaminations from polluted water. Adsorption of water contaminants by using suitable sorbents is one of the most commonly used methods for cleaning of water. Adsorbents with different characteristics and selectivity have been employed for the removal of heavy metals from water. For example, Maria et al. have used acrylonitrile (AN) based resin as a sorbent for the adsorption of heavy metals from aqueous medium.19 They prepared AN and divinylbenzene (DVB) copolymer beads, modified cyano groups of AN to amidoxime, amidrazone and oxazoline groups with the treatment of hydroxylamine, hydrazine and ethanolamine, respectively and investigated the effect of these modifications on the removal of heavy metal ions from water. Jencarova and Luptakova have reported biogenic iron sulphides prepared from sulphate-reducing bacteria (SRB) as a suitable sorbent for the removal of heavy metals from aqueous medium.20 Similarly another adsorbent having mesoporous nature has been introduced by Clercq for the selective removal of mercury from water.21 Removal of methylene blue and Rhodmine 6G from aqueous medium has been carried out by using graphene oxide as adsorbent.22 Graphene based composites has also been used as adsorbents for the removal of paraquat from water.23In comparison to other adsorbents, hydrogels are becoming more popular materials for the adsorption of contaminants from waters. Depending upon hydrophilic groups and extent of their hydrophilicity, degree of cross linking, ionic strength of solution, temperature and pH of the medium hydrogels can swell up to large volume as compared to their original volume in dry state.24 Hydrophilicity of hydrogels can be tuned by utilizing monomers having hydrophilic groups such as –OH, –COOH, –NH2, –CONH2, –SO3H.25 Due to ease of handling, biocompatibility, environmental friendly behavior and their reusability in case of development of efficient desorption method have made hydrogels very attractive materials for adsorption of pollutants like heavy metals, organic dyes and herbicides from aqueous medium. Previously our research group has reported removal of heavy metals from water with bulk hydrogels.26,27 Although, bulk gels are very easy to handle as they are used as adsorbents, there are some issues associated in their utilization that need to be overcome. For example, the adsorption rate is very slow due to their slow swelling rates; e.g., bulk gels take several hours to complete their adsorption task.26,27 On the other hand, microgels having particles size less than one micron can complete their adsorption task within few minutes, however, their practical usage, and handling is very difficult due to small dimensions.28 Additionally, after completion of adsorption task, the removal of microgels from the medium is very time consuming and could be costly and energy consuming: for example, for the microgels separation from reaction medium centrifugation maybe required. Therefore, there is a great need to prepare such type of hydrogels that can be readily handled, practical to use, and easily separable from the reaction mixture. So, to overcome issues, an alternate hydrogel was designed by Sahiner et al.28 by inserting microgels in bulk hydrogels to prepare microgel-bulk gel interpenetrating network (IPN). However, synthesis of such IPN is also time consuming process, and the adsorption time maybe longer in creation application. Therefore, the microgels that are about micrometer sizes can be considered as a good option to overcome the time consuming adsorption problems together with the magnetic properties may offer an alternative for handling and slow adsorption rate issues with practical applicability in real use for environmental application. In the present work, we have prepared p(MAc-co-AN) spherical micron sized hydrogel particles. The nitrile group of AN were modified to amidoxime groups by treating p(MAc-co-AN) microgels with hydroxyl ammonium chloride. Amidoximated microgel particles were used for the adsorption of heavy metals such as Cr(III) and Cd(II), and organic dyes as MB and R6G and a most frequently used herbicide, paraquat, from aqueous medium. Different parameters affecting the rate of absorption such as pH of solution, amount of microgels and the initial concentration of heavy metals were also investigated.
Experimental
Materials
Acrylonitrile (AN) and methacrylic acid (MAc, 99%, Aldrich) as monomers, N,N-methylenebisacrylamide (MBA 99%, Acros) as the crosslinking agent, ammonium persulfate (APS, Aldrich) as initiator, N,N,N′,N′-tetramethylethylenediamine (TEMED, Merck) as an accelerator, sorbitane monooleate (SPAN®80, Fluka) as a surfactant and cyclohexane (99.8%) used as solvent were purchased from Aldrich and used as received. Sodium hydroxide (NaOH, 98–100.5% Sigma Aldrich) and hydroxylamine hydrochloride (NH2OH·HCl, 98% Sigma Aldrich) were used for amidoximation. Cobalt(II) chloride hexahydrate (CoCl2·6H2O, 99% Sigma Aldrich) and iron(II) sulphate hexahydrate (Fe2SO4·7H2O, 99.5%, Merck) were used as metal ion sources while sodium borohydride (NaBH4, 98% Aldrich) was used as reducing agent for metal nanoparticle preparation. Methylene blue (MB, 97%, Fluka) and rhodamine 6G (R6G, Sigma) were used as organic dyes. Chromium(III) chloride hexahydrate (CrCl3·6H2O, 96%, Aldrich), cadmium(II) chloride hemipentahydrate (CdCl2, 98%, Fluka) as heavy metal ion sources and paraquat was used as herbicide. Hydrochloric acid (HCl, 36.5–38%, Sigma Aldrich) was used to adjust pH of metal ion solutions. Double distilled water (DDW) was used throughout the experiments.Synthesis of p(MAc-co-AN) microgels
Inverse suspension polymerization technique was adopted to prepare p(MAc-co-AN) microgels. Briefly, 100 mL cyclohexane and 320 μL span 80 were mixed in a 250 mL round bottom flask. This mixture was homogenized by rapid stirring and oxygen was removed from the mixture by N2 purging for 15 minutes. 0.0364 g of the cross-linker, MBA (0.5 mol% of monomers) and 0.2154 g of initiator, APS (2 mol% of monomers) were dissolved in 2 mL DDW in a vial by vortex mixer. 2 mL MAc and 1.548 mL of AN were also added in the same vial and the mixture was vortexed again to homogenize. The mixture of monomers, cross-linker and initiator was transferred to reaction flask already containing cyclohexane-span 80 mixture. This new reaction mixture was stirred for 10 minutes at 600 rpm continuously under N2 purging. Then the reaction was initiated by the addition of 0.5 mL TEMED and allowed to proceed in N2 atmosphere for 4 hours at 40 °C in an oil bath. The prepared microgel particles were collected by decantation of cyclohexane. In order to clean, the microgel particles were washed with ethanol and then with DDW by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 20 °C for 10 minutes followed by removal of the supernatant solution and re-dispersing in distilled water and re-centrifugation at least five times. Finally, p(MAc-co-AN) microgel particles were dried in oven at 60 °C for further use.
000 rpm and 20 °C for 10 minutes followed by removal of the supernatant solution and re-dispersing in distilled water and re-centrifugation at least five times. Finally, p(MAc-co-AN) microgel particles were dried in oven at 60 °C for further use.
Amidoximation reaction
In order to increase the hydrophilicity and metal ion absorption capacity of the prepared microgels, the hydrophobic nitrile (AN) groups were converted into hydrophilic amidoxime groups. This conversion was carried out by treating p(MAc-co-AN) microgels with hydroxylamine hydrochloride (NH2OH·HCl). In a typical reaction, in a reaction flask at least three fold excess (with respect to number of moles of AN in microgel) NH2OH·HCl was first neutralized by treating with equivalent amount of NaOH in 100 mL DDW. Then, certain amount of p(MAc-co-AN) microgel was added in reaction flask and reaction was allowed to proceed for 24 hours at 80 °C in an oil bath. At the end of this reaction time, the obtained amidoximated-poly(methacrylic acid-co-acrylonitrile) (amid-p(MAc-co-AN)) microgels were washed with DDW several times and then treated with NaOH in aqueous medium to deprotonate carboxyl groups of methacrylic acid units. After deprotonation microgels were again washed with DDW to remove excess NaOH and then dried at 60 °C in oven. These dried microgels were further used for characterization and as microreactors for the synthesis of metal nanoparticles. Amidoximation reaction was confirmed by Fourier Transformation Infra-Red (FT-IR, Thermo scientific, Nicolet iS 10) Spectrophotometer.Synthesis of magnetic microgel composites
Magnetic microgel composites were prepared by in situ fabrication of Co–Fe bimetallic nanoparticles in microgel network. In a typical synthesis procedure, metal ion solution was prepared by mixing 25 mL of 500 ppm solution of each of Co(II) and Fe(II) in DDW. About 0.1 g of amid-p(MAc-co-AN) microgel was added in that mixture and allowed to load metal ions for 2 hour under constant stirring at 400 rpm. Metal ion loaded microgels were allowed to settle down and then separated from the metal ion solution by decantation of solution and washed with DDW in order to remove loosely held metal ions. Finally, bimetallic magnetic nanoparticles were prepared inside the microgels by treating Co(II) and Fe(II) loaded microgels with 100 mL 0.1 M solution of NaBH4 under constant stirring at 400 rpm for three hours. The prepared magnetic microgel composites were washed with DDW and then acetone and dried in oven at 60 °C.Adsorption experiments
Adsorption experiments were carried out at room temperature from aqueous solutions of heavy metal ions, organic dyes and herbicide. A 100 mL, 250 ppm solution of each metal ions was added in separate beakers, pH was adjusted as 2, 3, 4, 5 and 6 by using 0.5 M HCl or 0.5 M NaOH aqueous solutions and was measured by Sartorius Documeter pH meter. Dried 50 mg of microgels was added in each solution, and the adsorption was allowed to take place at 500 rpm. Samples of 0.1 mL volumes were taken after specific interval of time, and diluted 60 times by adding DDW, and the amounts of metal ions was determined by Atomic Absorption Spectrophotometer (Thermo Scientific, ICE 3000 series, AAS). The effect of the amount of microgels on metal ion adsorption was investigated by using 0.025, 0.05, 0.075, 0.10 and 0.125 g of microgels to adsorb metal ions from 250 ppm, 100 mL solutions having pH 5 and 6 for Cr(III), and Cd(II), respectively. Additionally, in order to study the effect of concentration of metal ion solution, the adsorption experiments were conducted with 100 mL solution of different concentrations; 50, 100, 150, 200 and 250 ppm while keeping the amount microgels the same, 0.05 g, and constant pH of solutions: 5 for Cr(III) and 6 for Cd(II) were kept constant. For the adsorption of MB R6G and PQ, 0.05 g of microgel was added in 100 mL solution of each of MB (1.6 × 10−4 M), R6G (1 × 10−4 M) and PQ (100 ppm). The solutions were stirred at 500 rpm and 0.1 mL samples were withdrawn after specific time interval, diluted to certain ration (10 times for each of MB and R6G and 8 times for PQ) with DDW and their concentrations were measured by UV visible spectrophotometer (UV-Vis, T80+, PG Instruments) in terms of their maximum absorption at 664, 530, and 257 nm for MB, R6G and PQ respectively.Results and discussion
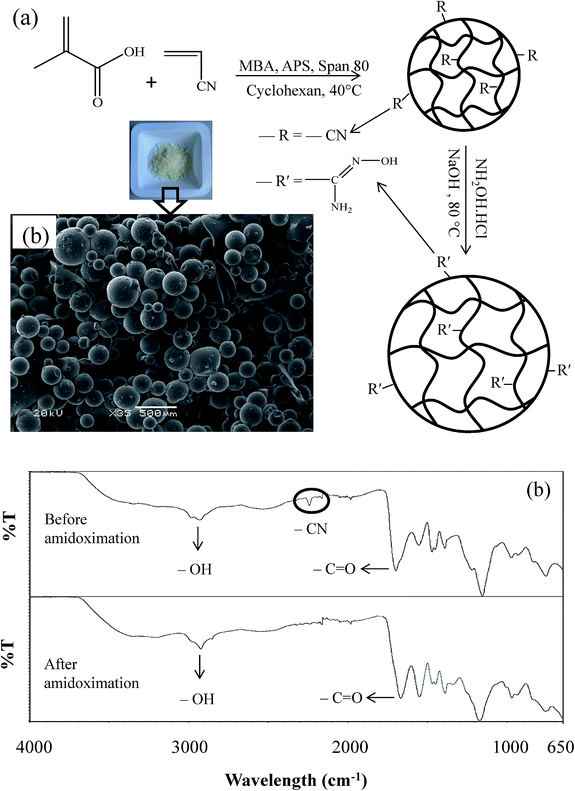
Synthesis and modification of p(MAc-co-AN) microgels
Schematic representation for the synthesis and modification of p(MAc-co-AN) microgels is shown in Fig. 1(a). Micron sized cross-linked spherical microgel particles were obtained by inverse suspension polymerization. Mole ratio of the MAc and AN was kept as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 0.5 mol% of the MBA, based on the total MAc and AN moles was used as cross linking agent to prepare cross linked microgels. SEM image of the prepared microgels is given in Fig. 1(b), which indicated that well define spherical microgel particle were obtained and most of the particles have diameters around 200 μm. As nitrile can be easily modified to more hydrophilic amidoxime groups which have greater tendency to adsorb heavy metal ions; so in order to increase the hydrophilic character and adsorption capacity of the prepared microgels, the nitrile groups on AN were modified into amidoxime groups by treating with hydroxylamine hydrochloride. This modification was confirmed by FT-IR spectroscopy. FT-IR spectra of p(MAc-co-AN) before and after modifications are given in Fig. 1(c). Before modification, nitrile peak can be clearly seen at 2241 cm−1 but after modification nitrile peak was disappeared showing the conversion of nitrile groups. The complete disappearance of nitrile absorption band is representing that almost all the nitrile groups were converted into amidoximes as in case of incomplete conversion, the nitrile band can be observed as a big decrease in the peak intensity.29,30 However, the presence of a broad peak at about 3000 cm−1 for –OH and another peak at 1700 cm−1 for –C
1, and 0.5 mol% of the MBA, based on the total MAc and AN moles was used as cross linking agent to prepare cross linked microgels. SEM image of the prepared microgels is given in Fig. 1(b), which indicated that well define spherical microgel particle were obtained and most of the particles have diameters around 200 μm. As nitrile can be easily modified to more hydrophilic amidoxime groups which have greater tendency to adsorb heavy metal ions; so in order to increase the hydrophilic character and adsorption capacity of the prepared microgels, the nitrile groups on AN were modified into amidoxime groups by treating with hydroxylamine hydrochloride. This modification was confirmed by FT-IR spectroscopy. FT-IR spectra of p(MAc-co-AN) before and after modifications are given in Fig. 1(c). Before modification, nitrile peak can be clearly seen at 2241 cm−1 but after modification nitrile peak was disappeared showing the conversion of nitrile groups. The complete disappearance of nitrile absorption band is representing that almost all the nitrile groups were converted into amidoximes as in case of incomplete conversion, the nitrile band can be observed as a big decrease in the peak intensity.29,30 However, the presence of a broad peak at about 3000 cm−1 for –OH and another peak at 1700 cm−1 for –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O before and after modification represents the presence of carboxylic groups of MAc moieties.
O before and after modification represents the presence of carboxylic groups of MAc moieties.
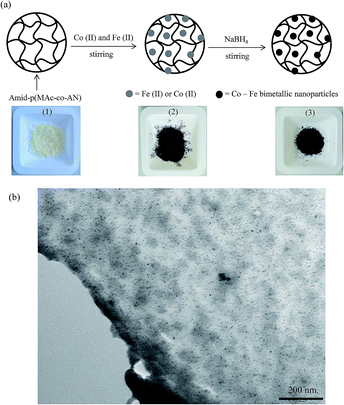
In situ synthesis of Co–Fe bimetallic magnetic nanoparticles
In the field of magnetic nano materials Co–Fe bimetallic nanoparticles have received much attention due to their high saturation magnetization (Ms), high Curie temperature, low coercivity, high permeability, low magnetocrystalline anisotropy, stability and superior magnetic properties.31 Because of the better magnetic properties, Co–Fe bimetallic nanoparticles have been considered as one the most suitable materials to incorporate magnetic character into polymeric structures or microgels in order to remove them from reaction medium by applying an external magnetic field as they are used as adsorbents. The high Ms values, superior magnetic properties and the introduction of an easy synthesis procedure was the motivation behind the synthesis of Co–Fe bimetallic nanoparticles. Co–Fe bimetallic nanoparticles were prepared inside the microgels by in situ reduction of both the loaded metal ions simultaneously. Schematic representation for the synthesis of bimetallic nanoparticle and physical appearance of reaction mixture at every step is shown in Fig. 2(a) via digital camera images. In Fig. 2(a), image 1 shows that before loading of metal ions microgels were colorless but upon loading of metal ions color of microgel was turned to brownish black that was developed due to mixing of pink color of Co(II) and brown color of Fe(II) as shown in image 2. After simultaneous reduction of both the metal ions with NaBH4 within microgel network, the color was turned to black as shown by digital camera image 3 in Fig. 2(a). TEM image of the Co–Fe bimetallic nanoparticles prepared in amid-p(MAc-AN) microgels is shown in Fig. 2(b). As can be seen there is no aggregation of nanoparticles and evenly distributed throughout the microgel matrices. The absence of aggregation reveals the ability of microgels to stabilize the in situ prepared nanoparticles within the network.The amount of metal nanoparticles in terms of their corresponding metal ions was measured by AAS. For this purpose the 0.05 g of the prepared composites were first treated with 5 M HCl (30 mL) to dissolve the entrapped metal nanoparticles within microgels into their corresponding metal ions and then amount of metal ions was measured by AAS. It was found that 61.87 mg (1.05 mmoles) of Co(II) and 63.89 mg (1.14 mmoles) of Fe(II) was present in per gram of dry microgels. The mole ratio between Co and Fe atoms in nanoparticle was measured from their corresponding amounts in mg and it was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
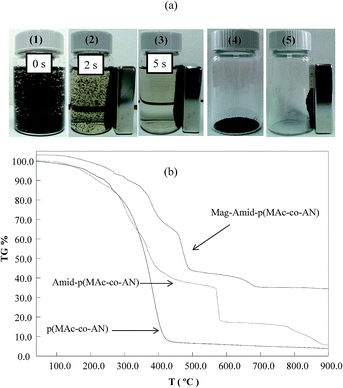
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1. Fig. 3(a) shows the magnetic behavior of the prepared microgel composites. As can be seen from image 1, in the absence of external magnetic field microgel composites remain suspended and can move freely in aqueous medium. However, as soon as an external magnetic field was applied, movement of composites was influenced and the composites were directed towards the magnet as shown in image 2, and within 5 seconds all the composite particles were attracted towards the magnet and attached firmly on the wall of container near the magnet, which can be clearly seen from image 3. Digital camera image of dried composites is also shown in image 4 that represents no change in color of microgel composites upon drying. As shown in image 5, the dried microgel composites were also attracted by externally applied magnet illustrates that these composites retain their magnetic behavior even after drying. This strong and durable magnetic behavior of the prepared composites is a very useful tool for their easy separation from reaction mixture on completion of their mission.
1.1. Fig. 3(a) shows the magnetic behavior of the prepared microgel composites. As can be seen from image 1, in the absence of external magnetic field microgel composites remain suspended and can move freely in aqueous medium. However, as soon as an external magnetic field was applied, movement of composites was influenced and the composites were directed towards the magnet as shown in image 2, and within 5 seconds all the composite particles were attracted towards the magnet and attached firmly on the wall of container near the magnet, which can be clearly seen from image 3. Digital camera image of dried composites is also shown in image 4 that represents no change in color of microgel composites upon drying. As shown in image 5, the dried microgel composites were also attracted by externally applied magnet illustrates that these composites retain their magnetic behavior even after drying. This strong and durable magnetic behavior of the prepared composites is a very useful tool for their easy separation from reaction mixture on completion of their mission.
Thermal properties of the prepared microgels and magnetic microgel composites were investigated by performing thermo gravimetric (TG) analysis. Thermograms of bare microgels before, and after amidoximation and of composite microgels are given in Fig. 3(b). As can be seen, bare p(MAc-co-AN) microgels undergo a single step degradation which was started around 100 °C and ended up at 428 °C with 92% weight loss. However, degradation rate was slow till 300 °C, but it became very high in the temperature range of 300 to 428 °C. After amidoximation of microgels, three steps degradation was observed. In the first step, 61% weight loss was observed in the temperature range of 100 to 457 °C. In the second step, 21% weight loss was observed in the temperature range of 568 to 581 °C, and in third step another 10% weight loss as occurred as temperature was increased from 783 to 900 °C. The difference in thermal properties of microgels before and after amidoximation can be attributed due to structural changes that were achieved by amidoximation reaction. In case of magnetic microgel composites, the degradation was occurred in four steps with a total 16, 36.6, 56, and 64% weight losses as the temperature was increased to 358, 453, 496 and 900 °C. The lesser weight loss and better thermal stability of magnetic microgel composites can be achieved due to strong coordination interaction between metal nanoparticle and amidoxime groups and carboxylate groups that are developed due to the presence of small amount of charge on the surface of metal nanoparticles.32
Adsorption studies
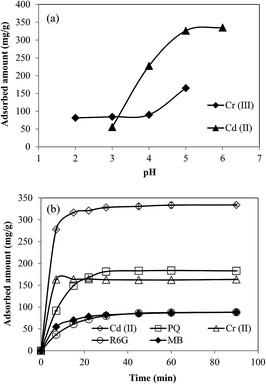
Adsorption ability of the prepared microgels and magnetic microgel composites was investigated as adsorbents for the removal of heavy metal ions, Cd(II), Cr(III), and organic dyes such as MB and R6G and a herbicide, PQ. As the ionization of Cd(II) and Cr(III) salts depends upon pH of the solution, the adsorption of these metal ions also depends on the solution pH. So, first of all the effect of pH on adsorption of metal ions was studied using 250 ppm 100 mL solution of both Cd(II) and Cr(III) that were prepared, and the pH of solution was adjusted from 3 to 6 for Cd(II) and 2 to 5 for Cr(III) solutions by the addition of 0.5 M HCl or NaOH aqueous solutions. At higher pH values, these ions were precipitated in the form of hydroxides whereas at very low pHs, the carboxylic groups of methacrylic acid groups become protonated that lead to contraction of microgel particles size, and hence adsorption capacity of microgels also become very low. Therefore, the effect of pH of solution on adsorption was study in the selected pH ranges as mentioned above, and the maximum amount of the metal ions, dyes, and herbicide adsorbed per gram of dry microgel was calculated by using mass balance equation which is given below| q = (C0 − Ce)V/W | (1) |
Upon amidoximation, the amidoxime groups also show similar protonation and deprotonation behavior favoring higher amounts of metal ion absorption at pHs below 7 (ref. 15, 27 and 28) and overall greater amount adsorbed of Cd(II) as compared to Cr(III) also shows that our microgels have greater tendency to adsorb Cd(II). Since maximum adsorption was observed at pH 5 for Cr(III) and pH 6 for Cd(II) so further experiments were carried out at these pH values. Kinetics of adsorption process was studied by taking samples from the adsorption medium after specific time intervals and concentrations of sample solutions were measured. Fig. 4(b) shows the time dependent adsorption of Cd(II), Cr(III), PQ, MB and R6G. It can be seen from Fig. 4(b) that for metal ions, maximum adsorption was achieved in 7 to 22 minutes, and for MB, R6G and paraquat maximum adsorption time was 30 minutes. The maximum amounts adsorbed per gram of dry amid-p(MAc-co-AN) microgels were 334 mg of Cd(II), 169 mg of Cr(III), 185 mg of PQ, 89 mg of R6G and 88 mg of MB. This adsorption capacity of these micron sized microgel particles for heavy metal ions is greater from the previous report of Masoumi and Ghaemy33 where they used amidoxime group containing nanohydrogels for the removal of heavy metal ions and adsorption capacity for Cd(II) and Cr(III) which were found as 66.67 and 71.42 mg g−1. The adsorption capacity for Cd(II) is remarkably greater from the previous results obtained by using some other adsorbents like peels of banana that were used by Anwar et al.34 for the removal of Cd(II) from water, and it was found that banana peels have adsorption capacity of only 5.71 mg g−1. Activated carbon prepared from coirpith was employed as adsorbent by Kadirvelu and Namasivayam,35 and maximum adsorption was found to be 93.4 mg g−1. The adsorption capacity of Cr(III) observed in the present is also very high as compare to previous reports where the authors have used lime stone,36 and modified polyacrylonitrile fibers37 as adsorbents. The amount of PQ adsorbed by amid-p(MAc-co-AN) microgels is also greater as compared to previous studies which were carried by using activated bleaching earth (maximum adsorption was 40.323 mg g−1)38 and clay.39 The results obtained for the removal of dyes were also superior from earlier work reported by Ayad et al.40 where they applied polyaniline nanotubes for the adsorption of MB and maximum adsorption capacity as around 5 mg g−1. The removal of MB was also studied by Zhang et al.41 by applying magnetic Fe3O4@C nanoparticle as sorbents, and it was observed that maximum 44.38 mg of MB was adsorbed per gram of the applied magnetic nanoparticles. So, the modified amid-p(MAC-co-AN) microgel composite are superior in many respect in comparison to the similar materials and can be used for variety of contaminant removal from aquatic media.
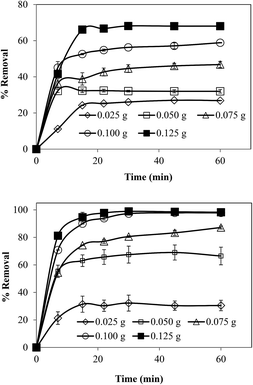
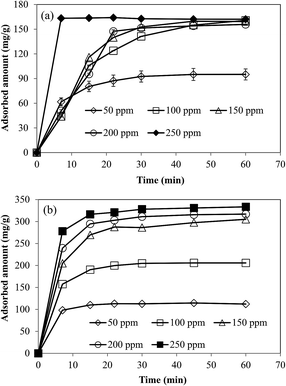
The effect of the amount of microgel was also investigated by using five different amounts of microgels as adsorbents while keeping all other parameters like the concentration, volume of solution, temperature and stirring rates constant. By increasing the amount of microgels from 0.025 to 0.125 g, the percent removal of metal ions was increased from 27 to 68% for Cr(III) and from 30 to 98% for Cd(II) as illustrated in Fig. 5(a) and (b) for Cr(II) and Cd(II), respectively. The increase in percent removal by increase in amount of microgel can be achieved due to increase in available adsorption sites. In order to determine the effect of initial concentration of metal ions on the adsorption capacity of the prepared microgels, the solutions of Cd(II) and Cr(III) at different concentrations (50, 100, 150, 200 and 250 ppm) were prepared and pH was adjusted at maximum adsorption value (pH 5 for Cr(II) and 6 for Cd(II)). And 0.05 g of microgels was used as adsorbent in 100 mL solution of these different metal ion concentrations. The amounts of metal ions adsorbed on microgels from DDW solutions of different concentration are shown in the form of adsorption isotherms in Fig. 6(a) and (b) for Cr(III) and Cd(II) respectively. As can be seen from Fig. 6, maximum adsorbed amount was increased with increase in initial concentration of metal ions in solutions. Since with the increase in metal ion concentration, amount of metal ions per unit volume was increased so greater number of metal ions was available to come in contact with microgels and hence their larger amount was adsorbed. For the adsorption of Cd(II) and Cr(III), the relationship between qe versus Ce was plotted and shown in Fig. 7(a). In order to determine the nature of adsorption followed by the adsorption of Cd(II) and Cr(III) on the prepared microgels, both the Langmuir and Freundlich adsorption isotherms were constructed. Langmuir adsorption isotherm describes the formation of monolayer of adsorbate on the surface of adsorbent by assuming that surface of the adsorbent is homogeneous and all adsorption sites are equivalent. Langmuir adsorption isotherm was constructed by using the following Langmuir equation.
| Ce/qe = Ce/qm + 1/qmKL | (2) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n KF + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce are given in Fig. 7(c) which show nonlinear pattern and confirms that the adsorption process did not follow Freundlich adsorption isotherm. Also, the values of 1/n are lesser than 1 representing that this adsorption process was not followed by Freundlich adsorption isotherm.
Ce are given in Fig. 7(c) which show nonlinear pattern and confirms that the adsorption process did not follow Freundlich adsorption isotherm. Also, the values of 1/n are lesser than 1 representing that this adsorption process was not followed by Freundlich adsorption isotherm.
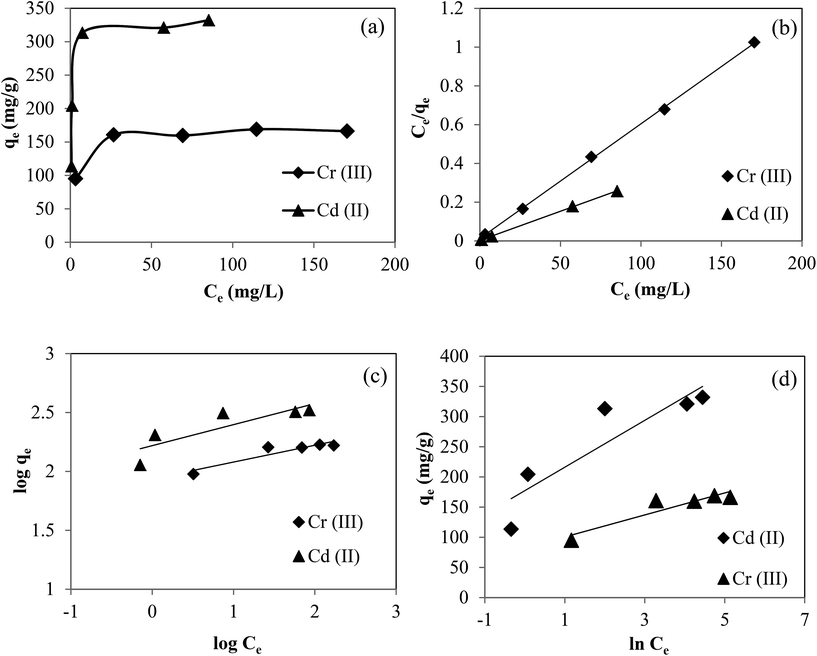 | ||
| Fig. 7 Plots of qe vs. Ce for the removal of Cr(III) and Cd(II). Application of (b) Langmuir isotherm (c) Freundlich isotherm and (d) Temkin isotherm for the adsorption of Cr(III) and Cd(II). | ||
Temkin adsorption isotherm that takes into account the effect of heat of adsorption as well as interaction between adsorbents and adsorbate was also applied by using the following equation.
qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KT + B KT + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (4) |
| B = RT/bT | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce as shown in Fig. 7(d). As indicated from R2 values (0.8063 and 0.8671 for Cd(II) and Cr(III)) which are given in Table 1 implies that Temkin isotherm did not provide a linear fit to experimental data. Therefore, from the best fit linear relation in case of Langmuir adsorption isotherms represent the best model for the adsorption of Cd(II) and Cr(III) by p(MAc-co-AN) based microgels.
Ce as shown in Fig. 7(d). As indicated from R2 values (0.8063 and 0.8671 for Cd(II) and Cr(III)) which are given in Table 1 implies that Temkin isotherm did not provide a linear fit to experimental data. Therefore, from the best fit linear relation in case of Langmuir adsorption isotherms represent the best model for the adsorption of Cd(II) and Cr(III) by p(MAc-co-AN) based microgels.
| Metal ion | Langmuir isotherm constants | Frenudlich isotherm constants | Temkin isotherm constants | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL (L g−1) | qm (mg g−1) | R2 | KF (L g−1) | n | R2 | KT (L g−1) | B | R2 | |
| Cd(II) | 0.447 | 333.3 | 0.9996 | 5.34 | 5.6 | 0.7279 | 95.9 | 38.85 | 0.8063 |
| Cr(III) | 1.034 | 169.5 | 0.9995 | 86.47 | 7.0 | 0.8558 | 92.1 | 18.23 | 0.8671 |
Adsorption tendency of amid-p(MAc-co-AN) for the removal of heavy metal ions, organic dyes and herbicide was from tap, river and seawater was also investigated.
Kinetic parameters calculated from the applied adsorption isotherms are given in Table 1. The amounts of metal ions, dyes and herbicide adsorbed by per gram of dry amid-p(MAc-co-AN) microgels are presented in Table 2.
| Contaminant | DDW | Tap water | River water | See water |
|---|---|---|---|---|
| a DDW = double distilled water, MB = methylene blue, R6G = rhodamine 6G, PQ = paraquat. | ||||
| Cd(II) | 333 | 318 | 187 | 95 |
| Cr(III) | 166 | 84 | 60 | 64 |
| MB | 88 | 96 | 00 | 03 |
| R6G | 90 | 46 | 10 | 21 |
| PQ | 190 | 59 | 5 | 00 |
The results show that prepared microgels can adsorb heavy metal ions from all types of waters, but the adsorption capacity was remarkably decreased in case sea and river waters that could be due to presence of other contaminants. As sea and river waters contain a lot of common dissolve metals such as iron, calcium, magnesium and so on, and contain many interfering species salts etc., that can be adsorbed by microgels or can decrease the swelling and adsorption capacity of microgels leading to a decrease in the adsorbed amount of metal ions. Amid-p(MAc-co-AN) microgels were also found to be able to remove R6G from all types of waters, but this microgel showed poor capacity in removal of MB and PQ from river and sea water but it can adsorb significant higher amounts of these contaminants from tap water. These results show that including DDW our adsorbent have good potential to remove heavy metal ions, cationic organic dyes and cationic herbicide from tap water and have also tendency to remove heavy metal ions from river and seawater containing a lot of interfering salts and other contaminations.
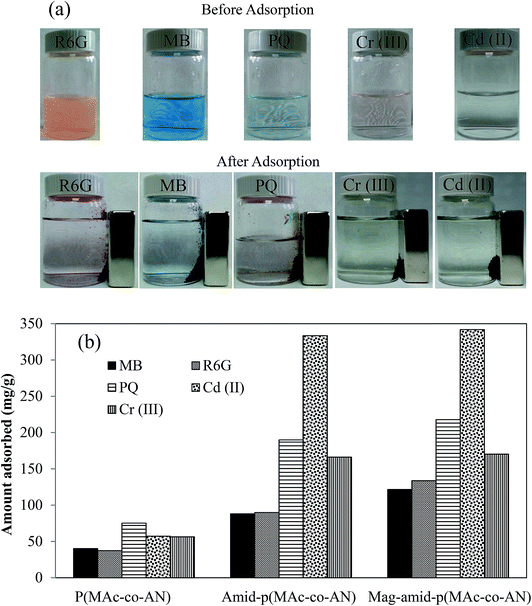
Adsorption capacity of magnetic microgels containing Co–Fe bimetallic magnetic particles was investigated by using 0.05 g of amid-p(MAc-co-AN) microgels loaded with magnetic nanoparticles to adsorb metal ions from 100 mL solution of metal ions (250 ppm), dyes (MB 1.6 × 10−4 M and R6G 1 × 10−5 M) and PQ (100 ppm). Fig. 8(a) represents digital camera images aqueous solutions of contaminants studied before the addition of microgels and after the completion of adsorption by added microgel composites. The decrease in intensities of the solution colours at the completion of adsorption process illustrates that contaminants were adsorbed on microgel composites. The advantage of magnetic properties of the prepared composites is illustrated in Fig. 8(a), which shows that after the completion of adsorption task, the magnetic composites were separated by applying external magnetic field. Attraction of almost all the added composites towards the externally applied magnet also shows that the prepared magnetic composites possess magnetic characteristics. In order to evaluate the effect of amidoximation of nitrile groups and magnetization of composites on the adsorption capacity of microgels, equivalent amounts of p(MAc-co-AN), amid-p(MAc-co-AN) and mag-amid microgels were used for the removal of metal ions, dyes and herbicide from their corresponding aqueous solutions under the similar experimental conditions. The results are presented in Fig. 8(b) that shows that adsorption capacity of amid-p(MAc-co-AN) was larger than that of p(MAc-co-AN) microgels. The increase in adsorption capacity of microgels after amidoximation was achieved due to conversion of nitrile groups into amidoxime groups. Adsorption capacity of the microgels was increased to 88.1, 89.9, 190.0, 334.5 and 166.5 mg g−1 from 40.2, 37.4, 75.3, 57.4, and 56.3 for MB, R6G, PQ, Cd(II), and Cr(III) respectively. The capacity increase can be due to the enhanced hydrophilicity of microgels by amidoximation that can also result in the increase of size of microgel particles.42 The increase in size of microgels increases the available space for the adsorbed species in microgel network and decreases the resistance in the diffusion of adsorbates by expanding the microgel network. Beside the increase in hydrophilic character, the amidoxime groups have also greater tendency to attract the heavy metal ions.43
Therefore, the amidoximation reaction brings three changes in microgel structure, the introduction of amidoxime groups, the increase in available space for the adsorbate species and the decrease in resistance against the diffusion of adsorbates by expanding the structure, and the new functional groups (amidoxime) to increase the adsorption capacity of microgel. Interestingly, after fabrication of magnetic nanoparticles within microgels, a slight increase in the adsorption capacity that were 121.6, 133.7, 217.8, 341.9, and 170.3 mg g−1 for MB, R6G, PQ, Cd(II), Cr(III), respectively. This slight increase in adsorption capacity of these toxic contaminants can be attributed to the possible interactions between the bimetallic nanoparticles present within microgels and positively charged adsorbates. Such an increase in metal ion adsorption capacity was also observed in an earlier work.26 So, the fabrication of magnetic nanoparticles not only offers easy removal of these adsorbents by applying external magnetic field upon the completion if adsorption task but also enhance the adsorption capacity of microgels. In order to evaluate the reusability of the prepared magnetic microgels, after adsorption process, the microgel composites were separated from adsorption medium and were treated with hydrochloric acid, sulphuric acid, nitric acid and carboxylic acid and also with the solution of NaOH separately, for the desorption of the adsorbed pollutants. Unfortunately, no significant desorption was observed. It can be due to very strong complexation of the used pollutants with polymeric microgel network. However, due to the ease of handling as compared to nano or smaller sized microgels, and rapid adsorption rate as compared to bulk gels, the prepared micron sized microgels can be sacrificially used as non-reusable adsorbents or for some other analytes can be reused many times such as the other common metal ions and aromatic nitro compounds and so on.26–29
Conclusions
Micron sized p(MAc-co-AN) microgel particles were prepared by inverse suspension polymerization and nitrile groups were successfully modified to amidoxime groups. The prepared microgels were used as sorbents for the removal of different types of contaminations from aqueous medium. Sorption capacity of microgels was increased after amidoximation of nitrile groups. From kinetic studies, it was observed that these microgels can adsorb maximum amount of heavy metal ions within 7 to 22 minutes, organic dyes and herbicide in 30 minutes. Higher removal efficiency and adsorption capacity for Cd(II) was observed as compare to Cr(III) however; the adsorption capacity for organic dyes was equivalent for both MB and R6G. The amid-p(MAc-co-AN) microgels were also found to be able to remove Cd(II), Cr(III) and R6G from tap, river and sea waters, however, the adsorption capability of MB and PQ from river and seawater was found to be negligible. Langmuir and Freundlich adsorption isotherms were applied on the adsorption of Cr(III) and Cd(II), and it was found that adsorption of these metal ions follows Langmuir adsorption model. Amidoximated microgels were also successfully used as templates in the synthesis of bimetallic magnetic nanoparticles. Magnetic microgels were also found to be able to remove heavy metal ions, organic dyes and herbicide from water. It was also demonstrated that magnetic properties of bimetallic nanoparticles containing microgels were retained even after adsorption of heavy metal ions, dyes and herbicide. So these microgels can be used as versatile adsorbents for the quick removal of a variety of contaminations from various waters whilst their micron size and magnetic behaviour offers their easy removal from the water upon the completion of adsorption task.Acknowledgements
This project was supported by financial support from The Scientific and Technological Research Council of Turkey (110T642). The author M. Ajmal is also grateful to The Scientific and Technological Research Council of Turkey (TUBITAK) for the support under the TUBITAK 2216 research fellowship program.References
- M. I. Lone, Z. He, Z. P. J. Stoffella and X. Yang, J. Zhejiang Univ., Sci., B, 2008, 9, 210 CrossRef CAS PubMed.
- S. Ahmed, S. A. Wahab and F. A. Marikar, Cent. Eur. J. Eng., 2012, 2, 304 Search PubMed.
- M. K. Bhatnagar, S. Raviraj, G. Sanjay and B. Prachi, J. Environ. Res. Dev., 2013, 8, 56 CAS.
- X. Bi, X. Feng, Y. Yang, G. Qiu, G. Li, F. Li, T. Liu, Z. Fu and Z. Jin, Environ. Int., 2006, 32, 883 CrossRef CAS PubMed.
- L. Järup, Br. Med. Bull., 2003, 68, 167 CrossRef PubMed.
- C. Garbisu and I. Alkorta, Bioresour. Technol., 2001, 77, 229 CrossRef CAS.
- C. Gisbert, R. Ros, A. Haro, D. J. Walker, M. P. Bernal, R. Serrano and J. N. Avino, Biochem. Biophys. Res. Commun., 2003, 303, 440 CrossRef CAS.
- M. Zendehdel, A. Barati, H. Alikhani and A. Hekmat, Iran. J. Environ. Health Sci. Eng., 2010, 7, 423 Search PubMed.
- S. Y. Mak and D. H. Chen, Dyes Pigm., 2004, 61, 93 CrossRef CAS PubMed.
- V. R. Kinhikar, Res. J. Chem. Sci., 2012, 2, 6 CAS.
- T. Ahmad, M. Rafatullah, A. Ghazali, O. Sulaiman, R. Hashim and A. Ahmad, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2010, 28, 231 CrossRef CAS PubMed.
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 2014, 209, 172 CrossRef CAS PubMed.
- S. H. Jang, G. Y. Jeonga, B. G. Mina, W. S. Lyoob and S. C. Lee, J. Hazard. Mater., 2008, 159, 294 CrossRef CAS PubMed.
- H. Zhao and H. Mitomo, J. Appl. Polym. Sci., 2008, 110, 1388 CrossRef PubMed.
- N. Sahiner, N. Pekel and O. Guven, Radiat. Phys. Chem., 1998, 52, 271 CrossRef CAS.
- S. Chen, Y. Zou, Z. Yan, W. Shen, S. Shi, X. Zhang and H. Wang, J. Hazard. Mater., 2009, 16, 1355 CrossRef PubMed.
- W. L. Yan and R. Bai, Water Res., 2005, 39, 688 CrossRef CAS PubMed.
- C. Liu, R. Bai and L. Hong, J. Colloid Interface Sci., 2006, 303, 99 CrossRef CAS PubMed.
- L. C. S. Maria, C. V. M. Amorim, R. M. P. M. P. Aguiar, I. C. Guimaraes, M. A. S. Costa, A. P. Aguiar, P. R. Rezende, M. S. Carvalho, F. G. Barbosa, J. M. Andrade and R. C. C. Ribeiro, React. Funct. Polym., 2001, 49, 133 CrossRef.
- J. Jencarova and A. Luptakova, Chemical Engineering Transactions, 2012, 28, 205 Search PubMed.
- J. D. Clercq, Int. J. Ind. Chem., 2012, 3, 1 CrossRef.
- S. T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2011, 359, 24 CrossRef CAS PubMed.
- Y. Tang, G. Zhang, C. Liu, S. Luo, X. Xu, L. Chen and B. Wang, J. Hazard. Mater., 2013, 252–253, 115 CrossRef CAS PubMed.
- R. Hernandez and C. Mijangos, Macromol. Rapid Commun., 2009, 30, 176 CrossRef CAS PubMed.
- J. M. Chern, W. F. Lee and M. Y. Hsieh, J. Appl. Polym. Sci., 2004, 92, 3651 CrossRef CAS PubMed.
- O. Ozay, S. Ekici, Y. Baran, N. Aktas and N. Sahiner, Water Res., 2009, 43, 4403 CrossRef CAS PubMed.
- D. Saraydin, Y. Isikver and N. Sahiner, Polym. Bull., 2001, 47, 81 CrossRef CAS.
- N. Sahiner, O. Ozay and N. Aktas, Chemosphere, 2011, 85, 832 CrossRef CAS PubMed.
- N. Pekel, N. Sahiner and O. Guven, Radiat. Phys. Chem., 2000, 59, 485 CrossRef CAS.
- K. Saeed, S. Haider, T. J. Oh and S. Y. Park, J. Membr. Sci., 2008, 322, 400 CrossRef CAS PubMed.
- M. Castrillon, A. Mayoral, A. Urtizberea, C. Marquina, S. Irusta, J. G. Meier and J. Santamarıa, Nanotechnology, 2013, 24, 505702 CrossRef CAS PubMed.
- H. H. Patterson, R. S. Gomez, H. Lu and R. L. Yson, Catal. Today, 2007, 120, 168 CrossRef CAS PubMed.
- A. Masoumi and M. Ghaemy, Carbohydr. Polym., 2014, 108, 206 CrossRef CAS PubMed.
- J. Anwar, U. Shafique, W. Zaman, M. Salman, A. Dar and A. Anwar, Bioresour. Technol., 2010, 101, 1752 CrossRef CAS PubMed.
- K. Kadirvelu and C. Namasivayam, Adv. Environ. Res., 2003, 7, 471 CrossRef CAS.
- H. A. Aziz, M. N. Adlan and K. S. Ariffin, Bioresour. Technol., 2008, 99, 1578 CrossRef CAS PubMed.
- B. Bagheri, M. Abdouss, M. M. Aslzadeh and A. M. Shoushtari, Iran. Polym. J., 2010, 19, 911 CAS.
- W. T. Tsai, C. W. Lai and K. J. Hsien, Chemosphere, 2004, 55, 829 CrossRef CAS PubMed.
- M. S. F. Santos, G. Schaule, A. Alves and L. M. Madeira, Chem. Eng. J., 2013, 229, 324 CrossRef CAS PubMed.
- M. M. Ayad and A. A. El-Nasr, J. Phys. Chem. C, 2010, 114, 14377 CAS.
- Z. Zhang and J. Kong, J. Hazard. Mater., 2011, 193, 325 CrossRef CAS PubMed.
- N. Sahiner and P. Ilgin, Polymer, 2010, 51, 3156 CrossRef CAS PubMed.
- X. Liu, H. Chen, C. Wang, R. Qu, C. Ji, C. Sun and Y. Zhang, J. Hazard. Mater., 2010, 175, 1014 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |