Fouling behavior of polyethersulfone ultrafiltration membranes functionalized with sol–gel formed ZnO nanoparticles†
Abstract
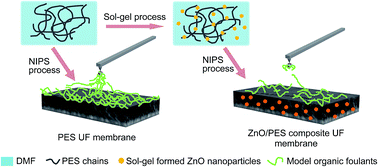
Ultrafiltration (UF) is an emerging membrane-based water separation process with potential application in drinking water treatment and for wastewater reuse. Nevertheless, membrane fouling decreases membrane performance and increases the frequency and cost of chemical cleaning. In this work, we present a method for loading ZnO nanoparticles into a polyethersulfone (PES) UF membrane to improve its fouling resistance. ZnO nanoparticles were synthesized with a sol–gel process using cost-effective precursors, resulting in particles with an average radius of 10 nm and ZnO content between 0.25 and 0.75 wt%. The hydrophilicity of the membrane surface was improved by the integration of ZnO nanoparticles, leading to a reduction in membrane contact angle (75.5 to 62.6°) and an increase in permeability (46.4 to 365.8 L m−2 h−1). Sodium alginate (SA), bovine serum albumin (BSA) and humic acid (HA) were chosen as model organic foulants to investigate fouling behavior of the fabricated membranes. Reduced fouling was conspicuously observed for ZnO/PES composite membranes compared to control membranes in all fouling cases. In order to better understand the fouling mechanism, atomic force microscopy (AFM) was used to quantify the intermolecular adhesion forces between the foulant and the clean or fouled membrane. Lower adhesion forces were observed for the modified membranes, indicating that the addition of sol–gel formed ZnO nanoparticles endows the PES UF membrane with an improved antifouling performance.

 Please wait while we load your content...
Please wait while we load your content...