Sensitive proteolysis assay based on the detection of a highly characteristic solid-state process
Hua Fan,
Jiantao Kou,
Dongdong Han,
Ping Li,
Dong Zhang,
Qiao Wu and
Qiang He*
Department of Hepatobiliary Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, P. R. China. E-mail: qianghebj@126.com; Fax: +86-10-85231503
First published on 26th May 2015
Abstract
This paper reported a sensitive proteolysis assay based on the detection of a highly characteristic solid-state process. In this study, acetylated peptides at the N-terminal were employed as the blockers of electrochemical signals. Silver nanoparticles (AgNPs) were used as electrochemical species which provided significant stripping current peaks. In the presence of proteases, the designed peptides could be cleaved and released amino groups which interacted with AgNPs and located the electrochemical species on the electrode. By detecting the linear sweep voltammetry, proteolysis processes could be sensitively monitored. We took trypsin and caspase-3 as two examples of proteases. This biosensing platform performed well towards the detection of both proteases. The detection limit for trypsin was 0.2 ng mL−1, and 0.1 pg mL−1 for caspase-3. Moreover, by changing the sequences of the substrate peptide, this method could be applied to detect different proteases. The utility in real samples was also checked with satisfactory results. Therefore, this method might have great potential use for the analysis of proteolysis and could be applied in clinical diagnostics.
Introduction
Proteases are a kind of enzyme that catalyze the hydrolytic degradation of proteins or peptides.1,2 They have been extensively studied in terms of upstreaming activating signals, signal transduction mechanisms and downstream regulations.3–5 For example, trypsin is a serine protease, produced in the pancreas as inactive protease trypsinogen, which can self-cleave to generate the active form.6 It plays an important role in the control of pancreatic exocrine functions and can be used as a specific diagnostic biomarker for pancreatic cancer.7 Caspase-3 belongs to cysteine-aspartic proteases family. It takes part in both intrinsic and extrinsic apoptosis, a highly regulated biological process that is associated with the fate of cells.8,9 The level of caspase-3 can be used as reliable target to detect apoptosis,10 which can then indicate the abnormal pathogeny and chemotherapy responses for a number of diseases including neurological disorders, autoimmune diseases and cancers.11–13 Sensitive detection of the proteases not only supports basic biology researches, but also helps the clinical diagnosis of certain diseases. Therefore, numerous analytical methods have developed, including western blot detection,14 colorimetric,15,16 fluorescent,17,18 and electrochemical methods.19–21 Nevertheless, applications of current approaches may be hindered because of certain drawbacks, e.g., laborious operations, complicated equipments and expensive reagents.In this study, we propose a simple and sensitive detection method for proteolysis assay based on the detection of the highly characteristic Ag/AgCl solid-state process. Trypsin and caspase-3 are chosen as two examples of proteases. Two substrate peptides are designed to be cleaved by the above proteases. The C terminal cysteine provides sulfhydryl group for the attachment on gold electrode surface22 and the acetylated N terminal is used to block electrochemical signals from silver nanoparticles (AgNPs).23 Since the peptides can be hydrolyzed by corresponding proteases, two segments of the peptides are created and the one with cysteine terminal remained on the electrode contains an amino group, which interacts with AgNPs, and a highly characteristic Ag/AgCl solid-state process is provided. Thereby, significant electrochemical signals can be obtained and the level of protease can be concluded. This method is quite simple but achieves high sensitivity. The detection limit for trypsin is 0.2 ng mL−1, and 0.1 pg mL−1 for caspase-3. Moreover, by changing the sequences of substrate peptide, this method can be applied to detect different proteases.
Experimental
Materials and chemicals
Trypsin, bovine serum albumin (BSA), thrombin, hemoglobin, glucose oxidase, Tris–HCl, silver nitrate (AgNO3), trisodium citrate, sodium borohydride (NaBH4), 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate (CHAPS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), tris(2-carboxyethyl)phosphine hydrochloride (TCEP), and DL-dithiothreitol (DTT) were purchased from Sigma (USA). Recombinant human caspase-3 (E. coli-derived) was ordered from R&D Systems, Inc. (Minneapolis, MN, USA). All the other chemicals were of analytical grade. Serum samples were from patients in Beijing Chaoyang Hospital. Water used to prepare all solutions was purified with a Milli-Q purification system (18 MΩ cm). Peptides were synthesized and purified by Sangon Biotech Co., Ltd. (Shanghai, China). P1 and P2 were acetylated at N-terminal. The sequences were as follows:P1: Ac-DGADRGGC
P2: Ac-DGADAGGC
Electrode preparation and stepwise modification
The substrate gold electrode was previously cleaned by piranha solution (98% H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% H2O2 = 3/1) for 3 min so as to remove any adsorbed material. Then, it was carefully polished on P5000 sand paper and 1, 0.3, 0.05 μm alumina slurry, respectively. After that, the electrode was sonicated in ethanol and then in water. Subsequently, the electrode was electrochemically cleaned with 0.5 M H2SO4. Before modification, the electrode was dried by purging with nitrogen.
30% H2O2 = 3/1) for 3 min so as to remove any adsorbed material. Then, it was carefully polished on P5000 sand paper and 1, 0.3, 0.05 μm alumina slurry, respectively. After that, the electrode was sonicated in ethanol and then in water. Subsequently, the electrode was electrochemically cleaned with 0.5 M H2SO4. Before modification, the electrode was dried by purging with nitrogen.
Peptide monolayer was self-assembled by incubating the electrode in 0.3 mL peptide solution (2 μM, 10 mM HEPES, 10 mM TCEP, pH 7.0) for 12 h at room temperature. TCEP could prevent terminal cysteine of the peptides from forming disulfide bonds. The peptide modified electrode was then treated with 0.1 mM MCH for 1 h to achieve well-aligned monolayers.
Synthesis of AgNPs
AgNPs were synthesized as follows. Mixture of 0.25 mM AgNO3 and trisodium citrate was prepared. Then, 10 mM NaBH4 solution was prepared for further borohydride reduction of AgNO3. 3 mL of NaBH4 was added to 100 mL of AgNO3 solution under vigorous stirring for 30 min. During the process, AgNPs were formed, which were purified by three cycles of centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min.
000 rpm for 20 min.
Proteases digestion and AgNPs attachment
Trypsin and caspase-3 catalyzed cleavage of corresponding peptides were performed as follows. The solutions of two proteases were prepared with desired concentrations in the reaction buffer (20 mM HEPES, 10 mM DTT, pH 7.5). The peptide modified electrode was then immersed in the solutions at 37 °C for 1 h. Then, the electrode was rinsed and treated with 10 nM AgNPs for another 1 h before further electrochemical detection. For comparison, interfering proteins including BSA, thrombin, hemoglobin, and glucose oxidase were used to digest the peptide and the electrochemical signals were recorded.Real sample assay
Complex biological fluids were checked to demonstrate the utility of this biosensor in real samples. Different amount of trypsin was spiked in serum samples before electrochemical detection. For caspase-3, apoptotic human pulmonary carcinoma A549 cells were lysed and the protease concentration was measured in the lysate. Briefly, A549 cells was cultured in DMEM supplemented with 10% fetal bovine serum at 37 °C until 90% confluence. Then, the cells were treated with Apoptosis Inducer Kit to induce apoptosis for 12 h. Afterwards, the cells were harvested, washed and lysed. The lysates were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and the supernatant was used for electrochemical detection.
000 rpm for 20 min and the supernatant was used for electrochemical detection.
Electrochemical measurement
Electrochemical impedance spectroscopy (EIS) and linear sweep voltammetry (LSV) were performed on a model 660d electrochemical analyzer (CH Instruments, Shanghai, China) with a conventional three-electrode cell. Saturated calomel electrode (SCE) was employed as the reference electrode, platinum-plate electrode was used as the counter electrode and the working electrode was the peptide modified gold electrode. EIS data was obtained by applying 5 mV amplitude within the frequency from 1 to 100 kHz. The electrolyte was 5 mM K4[Fe(CN)6]/K3[Fe(CN)6] containing 0.1 M KCl. The Ag/AgCl solid-state process was represented by LSV, which was performed in 0.1 M KCl with the scan rate of 100 mV s−1.Results and discussion
The sensing mechanism is depicted in Fig. 1. Cysteine ended peptide is attached on the electrode via the thiol–gold interaction.24,25 Then, MCH is used to block any unspecific adsorption and maintain the well-aligned peptide monolayer.26 The acetylated N-terminal cannot interact with AgNPs. However, after proteolysis process, the exposed amino group adsorbs AgNPs on the electrode surface. Afterwards, the Ag/AgCl solid-state process is carried out and significant electrochemical signals are obtained. Protease concentration can be figured out by analysing the electrochemical curves.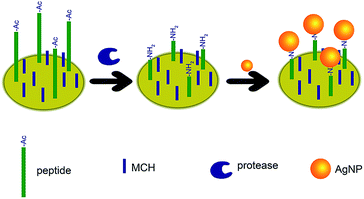 | ||
| Fig. 1 Schematic illustration of the electrochemical biosensor for the detection of proteolysis process. | ||
Characterization of the modification of electrode
We have checked the interface properties of the modified electrode by EIS. Fig. 2 shows the Nyquist plots of EIS for the electrode at different modification stages. The semicircular portion in the impedance spectrum indicates the interfacial charge transfer resistance. Bare gold electrode includes almost no semicircle portion. After the modification of peptide, a semicircle is observed due to the repellence of the redox species [Fe(CN)6]3−/4−. After further backfill of MCH, the semicircle domain grows much larger. However, the cleavage of peptide by proteases can decrease the hindrance between [Fe(CN)6]3−/4− and the electrode. Thus the semicircle domain gets smaller. LSV can also confirm the sensing strategy. Fig. 3 depicts the linear sweep voltammograms of bare electrode, peptide modified electrode, trypsin treated peptide modified electrode after the incubation of AgNPs. Without the proteolysis process, no amino groups can be released on the electrode surface, and no AgNPs can be localized. Therefore, only the electrode with proteolysis process reflect a significant silver stripping current peak. | ||
| Fig. 3 Linear sweep voltammograms of (a) bare gold electrode, (b) peptide modified electrode, (c) trypsin treated peptide modified electrode after the incubation of AgNPs. | ||
Quantitative determination of trypsin and caspase-3
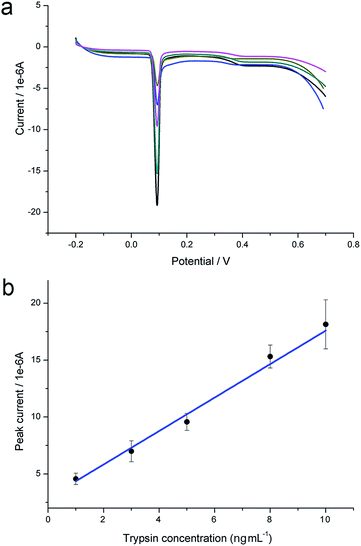
The LSV silver stripping peak is very sharp compared with traditional voltammetry curves and much suitable for quantitative analysis. Therefore, we take advantage of LSV current peak for the detection of trypsin and caspase-3. As show in Fig. 4a, larger amount of trypsin cleaves more acetylated peptides and the stripping peak current gets larger. Fig. 4b is the calibration curve of LSV peak in response to trypsin concentration. For each concentration, the measurement has been repeated at least for three times independently. A linear relationship is established within the range from 1 to 10 ng mL−1 with the detection limit of 0.2 ng mL−1 (S/N = 3). The equation is y = 2.892 + 14.69x, in which y is the value of peak current, x is the concentration of trypsin, R2 = 0.983. We have also obtained similar results in the case of caspase-3-based proteolysis. The LSV curves are shown in Fig. 5a. The linear relationship between peak current and caspase-3 concentration is established in Fig. 5b. The equation is y = 1.567 + 0.5504x, in which y is the value of peak current, x is the concentration of caspase-3, R2 = 0.993. The linear range is from 0.5 to 50 pg mL−1. The detection limit is calculated to be 0.1 pg mL−1 (S/N = 3). The sensitivity of the proposed strategy is among the highest, compared with previous reported methods.15,17,21 Error bars of the detection of each concentration of proteases are small, demonstrating high repeatability of the biosensor. The modified electrode is highly stable. After storing at 4 °C for 1 month, it still works well.Selectivity
To confirm the selectivity of this biosensor for the detection of proteolysis. Different kinds of proteins including BSA, thrombin, hemoglobin, and glucose oxidase are explored. As shown in Fig. 6, the control proteins can be successfully distinguished from target proteases, which demonstrate the high selectivity of proteolysis assay. Three independent experiments are performed. | ||
| Fig. 6 LSV peak currents of interfering proteins (1 ng mL−1) compared with (a) trypsin (1 ng mL−1) and (b) caspase-3 (50 pg mL−1). | ||
Real sample assay
We have then performed the trypsin assay in human serum samples. The results are listed in Table 1. Different amount of trypsin has been spiked in the samples and the final concentrations are calculated according to the electrochemical signals and the regression equation of the standard curve. The recoveries and relative errors have confirmed satisfactory accuracy and precision of this proteolysis assay method. Caspase-3 level in apoptotic cells are detected and compared with normal cells. The distinct contrast in Fig. 7 is with good accordance to the state of cells27 and demonstrate the good utility of this capspase-3 assay method. Three independent experiments are performed.| Samples | Added (ng mL−1) | Detected (ng mL−1) | Recovery (%) | Relative error (%) |
|---|---|---|---|---|
| 1 | 2 | 2.4 | 120.0 | 3.5 |
| 2 | 4 | 4.4 | 110.0 | 5.2 |
| 3 | 6 | 6.9 | 115.0 | 4.3 |
 | ||
| Fig. 7 LSV peak currents response to the cell lysate with and without the treatment of Apoptosis Inducers Kit. | ||
Conclusions
In conclusion, we develop a strategy for proteolysis assay based on the detection of a highly characteristic solid-state process. Two proteases are checked. One is trypsin, a useful biomarker for pancreatic cancer; the other is caspase-3, a powerful apoptosis indicator. Both of them are important proteases involved in cellular processes, which have great clinical significance. Excellent analytical performances are obtained by designing two peptide modified electrode. This biosensor is high sensitive and selective. Moreover, by changing the sequences of substrate peptide, this method can be applied to detect different proteases. Real samples can also be applied, which promise great potential use in clinical diagnostics in the future.Notes and references
- M. Yildiz, S. Ghosh, J. A. Bell, W. Sherman and J. A. Hardy, ACS Chem. Biol., 2013, 8, 2744–2752 CrossRef CAS PubMed.
- K. J. Son, D. S. Shin, T. Kwa, Y. D. Gao and A. Revzin, Anal. Chem., 2013, 85, 11893–11901 CrossRef CAS PubMed.
- R. Chaba, B. M. Alba, M. S. Guo, J. Sohn, N. Ahuja, R. T. Sauer and C. A. Gross, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2106–2111 CrossRef CAS PubMed.
- S. E. Ades, L. E. Connolly, B. M. Alba and C. A. Gross, Genes Dev., 1999, 13, 2449–2461 CrossRef CAS.
- E. P. Lu, M. McLellan, L. Ding, R. Fulton, E. R. Mardis, R. K. Wilson, C. A. Miller, P. Westervelt, J. F. DiPersio, D. C. Link, M. J. Walter, T. J. Ley and T. A. Graubert, Blood, 2014, 124, 3887–3895 CrossRef CAS PubMed.
- M. Hirota, M. Ohmuraya and H. Baba, J. Gastroenterol., 2006, 41, 832–836 CrossRef CAS PubMed.
- T. Marchbank, A. Mahmood and R. J. Playford, Am. J. Physiol. Ren. Physiol., 2013, 305, F382–F389 CrossRef CAS PubMed.
- K. Mnich, L. A. Carleton, E. T. Kavanagh, K. M. Doyle, A. Samali and A. M. Gorman, Cell Death Dis., 2014, 5, e1202 CrossRef CAS PubMed.
- A. G. Daniel, E. J. Peterson and N. P. Farrell, Angew. Chem., Int. Ed., 2014, 53, 4098–4101 CrossRef CAS PubMed.
- S. Takano, S. Shiomoto, K. Y. Inoue, K. Ino, H. Shiku and T. Matsue, Anal. Chem., 2014, 86, 4723–4728 CrossRef CAS PubMed.
- C. B. Lu, W. M. Fu and M. P. Mattson, Neurobiol. Dis., 2001, 8, 194–206 CrossRef CAS PubMed.
- E. Frejlich, J. Rudno-Rudzinska, K. Janiszewski, L. Salomon, K. Kotulski, O. Pelzer, Z. Grzebieniak, R. Tarnawa and W. Kielan, Adv. Clin. Exp. Med., 2013, 22, 593–602 Search PubMed.
- P. V. Jacobo, M. Fass, C. V. Perez, S. Jarazo-Dietrich, L. Lustig and M. S. Theas, Cytokine, 2012, 60, 385–392 CrossRef CAS PubMed.
- R. Mintzer, S. Ramaswamy, K. Shah, R. N. Hannoush, C. D. Pozniak, F. Cohen, X. R. Zhao, E. Plise, J. W. Lewcock and C. E. Heise, PLoS One, 2012, 7, e30376 CAS.
- P. Miao, T. Liu, X. X. Li, L. M. Ning, J. Yin and K. Han, Biosens. Bioelectron., 2013, 49, 20–24 CrossRef CAS PubMed.
- Z. J. Zhou, L. Peng, X. Y. Wang, Y. Xiang and A. J. Tong, Analyst, 2014, 139, 1178–1183 RSC.
- R. Huang, X. J. Wang, D. L. Wang, F. Liu, B. Mei, A. M. Tang, J. Jiang and G. L. Liang, Anal. Chem., 2013, 85, 6203–6207 CrossRef CAS PubMed.
- J. Zhang, X. Wang, W. J. Cui, W. W. Wang, H. M. Zhang, L. Liu, Z. C. Zhang, Z. Li, G. G. Ying, N. Zhang and B. H. Li, Nat. Commun., 2013, 4, 2157 Search PubMed.
- P. Miao, B. D. Wang, K. Han and Y. G. Tang, Electrochem. Commun., 2014, 47, 21–24 CrossRef CAS PubMed.
- S. W. Zhou, T. T. Zheng, Y. F. Chen, J. J. Zhang, L. T. Li, F. Lu and J. J. Zhti, Biosens. Bioelectron., 2014, 61, 648–654 CrossRef CAS PubMed.
- R. P. Liang, X. C. Tian, P. Qiu and J. D. Qin, Anal. Chem., 2014, 86, 9256–9263 CrossRef CAS PubMed.
- P. Miao, J. Yin, L. M. Ning and X. X. Li, Biosens. Bioelectron., 2014, 62, 97–101 CrossRef CAS PubMed.
- J. Zhang, B. P. Ting, N. R. Jana, Z. Q. Gao and J. Y. Ying, Small, 2009, 5, 1414–1417 CrossRef CAS PubMed.
- Y. R. Xue, X. Li, H. B. Li and W. K. Zhang, Nat. Commun., 2014, 5, 4348 CAS.
- P. Miao, B. D. Wang, F. Y. Meng, J. Yin and Y. G. Tang, Bioconjugate Chem., 2015, 26, 602–607 CrossRef CAS PubMed.
- P. Miao, L. M. Ning, X. X. Li, P. F. Li and G. X. Li, Bioconjugate Chem., 2012, 23, 141–145 CrossRef CAS PubMed.
- Y. L. Pan, M. L. Guo, Z. Nie, Y. Huang, Y. Peng, A. F. Liu, M. Qing and S. Z. Yao, Chem. Commun., 2012, 48, 997–999 RSC.
| This journal is © The Royal Society of Chemistry 2015 |