Ligand–metal-drug coordination based micelles for efficient intracellular doxorubicin delivery†
Lan Bai,
Fei Song*,
Xiao-hui Wang,
Jiang-yong-quan Cao,
Xue Han,
Xiu-li Wang and
Yu-zhong Wang*
Center for Degradable and Flame-Retardant Polymeric Materials (ERCPM-MoE), College of Chemistry, State Key Laboratory of Polymer Materials Engineering, National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: songfei520@gmail.com; yzwang@scu.edu.cn
First published on 13th May 2015
Abstract
In the field of anticancer drug delivery, improving drug loading capacity of carriers and achieving efficient intracellular drug transportation simultaneously is very difficult but they are important issues for the development of chemotherapy. Herein, a reversible ligand–metal-drug coordination architecture responsive to pH was employed to construct a smart drug delivery system, in which the ligand and metal can be regarded as a harbor and an anchor to not only moor the drug (doxorubicin, DOX) but also send the cargo precisely on time. Based on the strategy, DOX loading content of the system could run up to 26.1%. Owing to the stability of coordination bonds at neutral conditions, premature drug leakage was extremely suppressed to lower than 5%, while an almost complete drug release was realized at pH 5.0 as a result of breakage of the bonds. Presence of the coordination interaction played a key role in controlled release of DOX, which followed the first-order kinetics model in the case of non-coordinated systems but the pseudo-second-order kinetics model in the case of coordinated systems. Moreover, the metal-coordinated system could effectively take DOX into HeLa cells, presenting a comparable cancer therapy effect to free DOX. This study established exploitation of coordination interaction to connect drugs and carriers as a promising way to meet urgent needs for chemotherapy.
Introduction
Owing to great threats of cancer to human health, recent advances have focused on how to defeat this powerful enemy of mankind. Despite an indispensable therapeutic method for cancer therapy, chemotherapy is still facing a series of problems, including poor bioavailability and low accumulation of anticancer drugs in tumor sites as well as their serious side effects toward healthy tissues; thus, significant progress has been made for drug delivery systems, especially for the intelligent ones with particularly safe and effective cancer therapy.1–5 To the best of our knowledge, most carriers have merely relied on hydrophobic interactions to encapsulate anticancer drugs, generally resulting in a relatively low drug loading content,6–8 which still keeps them far away from clinical applications. In order to achieve a high drug loading capacity, different non-covalent bonds, such as π–π stacking,9 electrostatic interaction10,11 and hydrogen bonding,12 have been introduced between carriers and drugs; however, these interactions are not so sensitive to tumor microenvironment that endosomal escape as well as efficacy of drugs is hard to ensure. Therefore, how to solve contradictions between the encapsulation and release of drugs is a key point for development of advanced drug carriers.On the other hand, intracellular pH value is found to be different between normal and cancer cells, i.e., about 7.4 in the former but as low as 5.0 in the latter.13,14 This leads us to consider exploring a non-covalent bond that can be reversibly formed and cleaved at different pH values to settle this “dispute.” Since metal coordination is generally pH-dependent, it has been widely used in the construction of drug delivery systems.15–18 We have previously found coordination interaction exists between metal ions (Cu2+ for instance) and a frequently used anticancer drug, doxorubicin (DOX), under neutral conditions but it disappears in acidic environments, which can contribute to a precise drug release in cancer cells.19 A further consideration is how to achieve a high drug capacity in virtue of the special interaction. It was worth noting that a concept, “ligand–metal-drug” coordination bonding architecture, has been employed recently to develop inorganic drug delivery carriers,20–23 but the strategy has never been involved in any polymeric material. Along this thinking, a ligand that could interact with Cu2+ only at neutral conditions is expected to be introduced onto a polymer chain, which can not only provide a harbor to moor drugs but also send the cargoes on time.
Histidine (His), a kind of amino acid with good biocompatibility and endosomal membrane-disruption activity, has demonstrated a great potential in drug delivery.24,25 The pKa of the amino group in the imidazole ring of His is about 6.1,26 which can endow carriers with a pH-dependent amphoteric property. Moreover, as reported previously, His can form coordination interactions with metals during some biomimetic processes,27–29 since an electron lone pair on the unsaturated nitrogen is offered from the imidazole ring. Considering the pH-responsive characteristic of His, its interaction with metal is anticipated to be broken in an acidic environment. Therefore, the “ligand–metal-drug” concept will be available for polymeric carriers with the help of His. In the present work, a type of ABC triblock copolymer, poly(ethylene glycol)-b-poly(2-hydroxyethyl methacrylate-Boc-histidine)-b-poly(styrene) (PEG-PBHE-PS) was synthesized by the reversible-addition fragmentation chain-transfer (RAFT) polymerization. Due to its amphiphilicity, the copolymer could self-assemble into micelles in water. At neutral conditions, DOX was encapsulated via not only the hydrophobic interaction with PS but also the coordination architecture of His-metal-DOX on pendant histidine groups, resulting in an efficient drug loading, whereas the interactions among drug, metal and ligand were broken under acidic conditions, leading to a fast intracellular release as well as a successful endosomal escape of DOX. In particular, differences of self-assembly behavior, size, drug loading capacity, in vitro release and cytotoxicity were investigated in detail for the drug delivery system with or without Cu2+-coordination.
Results and discussion
Synthesis of PEG113-PBHEm-PSn triblock copolymers
The RAFT polymerization method was used to synthesize the triblock copolymer, PEG113-PBHEm-PSn (Fig. 1). PEG113-CTA was employed to conduct a sequential polymerization of Boc-His-EMA (BHE) and styrene in DMF at 75 °C in the presence of AIBN. As confirmed by 1H NMR, GPC and FT-IR analyses (Fig. S1–S6†), PEG113-PBHEm diblock copolymers and PEG113-PBHEm-PSn triblock copolymers with well-defined structures were obtained. Composition of the copolymers was determined by comparing the peaks of PEG block (3.6–3.8 ppm)30,31 with those attributed to PBHE (∼8.0 ppm)32,33 and PS blocks (6.3–7.2 ppm), respectively. Although a monomodal GPC trace was detected for PEG113-PBHEm diblock copolymers, their PDI was a little high in terms of a living polymerization. This can be attributed to the slow living free-radical polymerization rate that was caused by low activities of RAFT end group grafted on the long PEG chain and BHE monomer with a large Boc-His side group.34,35 As a result, a high feeding of BHE monomer had to be involved to obtain a long PBHE chain, but the exorbitant feeding led to an unsatisfactory conversion rate of monomer (Table S1†). Compared with that, due to the weaker steric hindrance of styrene, its polymerization was easier to control and thus resultant triblock copolymers just showed a slight increase in PDI. To understand the relationship between scale of the “harbor” (content of His) and delivery efficiency of cargoes, a series of triblock polymers were synthesized containing different lengths of PBHE block chain. The resultant copolymers, PEG113-PBHE10-PS10, PEG113-PBHE20-PS10, PEG113-PBHE30-PS10 and PEG113-PBHE40-PS10, were named as P1, P2, P3 and P4, respectively.Confirmation of coordination architecture
To investigate whether the desired high drug loading capacity and efficient intracellular release can be realized, it is necessary to confirm the normal operation of His-Cu-DOX strategy. First of all, the coordination interactions among His, Cu2+ and DOX were evaluated by UV-Vis measurement. For the neutral aqueous solution of His/DOX, multiple peaks, which can be attributed to DOX, were observed within 400–600 nm (Fig. 2). Once an addition of Cu2+, a decreased absorbance as well as a red-shift of the maximum absorbance peak (π–π* electron transition of the anthraquinone portion of DOX) at 497 nm was detected, suggesting the formation of the coordination interaction between DOX and Cu2+.36 However, when this ternary mixture was placed into an acidic solution, the maximum absorbance peak went back to 497 nm, which indicated that Cu2+/DOX interaction was reversible upon changing pH value. Moreover, since the characteristic UV absorbance of imidazole group located in the wavelength range of 180–250 nm,37,38 its signals were easily interfered with by Cu2+ (strong UV absorbance within 200–235 nm, Fig. S7†), and thus the interaction between His and Cu2+ was hard to be confirmed by the method.As a further investigation, FT-IR analysis was conducted on the His/Cu2+ mixture treated under different pH values. No matter at which condition (pH 5.0 or 7.4), all samples presented two same characteristic peaks at 3137 and 1706 cm−1 (Fig. 3) corresponding to the protected primary amine and carbonyl groups,37 indicating that they were not involved in the coordination, whereas the peaks of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of imidazole determined at 1596 and 1485 cm−1 red-shifted to 1620 and 1510 cm−1, respectively, after introducing Cu2+ at neutral condition, which implied the complexation of His with Cu2+. Once in an acidic environment, nearly no difference was found for His and His/Cu2+, illustrating the interaction between them was also reversible. Another proof for the pH-dependent interaction provided by Raman measurement (Fig. 4) was that (1) an apparent band at 1596 cm−1 belonging to C4
N stretching vibration of imidazole determined at 1596 and 1485 cm−1 red-shifted to 1620 and 1510 cm−1, respectively, after introducing Cu2+ at neutral condition, which implied the complexation of His with Cu2+. Once in an acidic environment, nearly no difference was found for His and His/Cu2+, illustrating the interaction between them was also reversible. Another proof for the pH-dependent interaction provided by Raman measurement (Fig. 4) was that (1) an apparent band at 1596 cm−1 belonging to C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5 stretching vibration (Nτ–H, Nπ–Cu2+) of the imidazole ring was merely for His/Cu2+ at pH 7.4 rather than other samples; (2) a band at 1628 cm−1, which can be attributed to the cationic imidazolium form (Nτ–H, Nπ–H) of His was well observed for all samples except His/Cu2+ at pH 7.4; and (3) a characteristic band (Nτ, Nπ–H) at 1263 cm−1 of His was not found for His/Cu2+ at pH 7.4.39,40 Based on the above results, the “His-Cu-DOX” concept was confirmed to work successfully. As is well known, Cu2+ consists of a d9 electronic configuration and is able to form tetradentate chelates with imidazole.41–43 A possible mechanism was therefore proposed for the formation of His-Cu-DOX complex (Fig. 5).
C5 stretching vibration (Nτ–H, Nπ–Cu2+) of the imidazole ring was merely for His/Cu2+ at pH 7.4 rather than other samples; (2) a band at 1628 cm−1, which can be attributed to the cationic imidazolium form (Nτ–H, Nπ–H) of His was well observed for all samples except His/Cu2+ at pH 7.4; and (3) a characteristic band (Nτ, Nπ–H) at 1263 cm−1 of His was not found for His/Cu2+ at pH 7.4.39,40 Based on the above results, the “His-Cu-DOX” concept was confirmed to work successfully. As is well known, Cu2+ consists of a d9 electronic configuration and is able to form tetradentate chelates with imidazole.41–43 A possible mechanism was therefore proposed for the formation of His-Cu-DOX complex (Fig. 5).
Characterization of PEG113-PBHEm-PSn micelles
Because of the amphiphilicity of the triblock copolymers, they could self-assemble into micelles in water, among which the PEG and PS blocks steadily played roles of hydrophilic corona and hydrophobic core, respectively. Since the aqueous solubility of PBHE block chain can be improved at acidic conditions as a result of the protonation of the imidazole amine group,44 status of the block in the middle was changed from hydrophobic core at pH 7.4 to hydrophilic shell at pH 5.0. CMC of PEG113-PBHEm-PSn was therefore decreased with the increase in length of the PBHE chain (Fig. S8†). Furthermore, with the addition of Cu2+, a lower CMC was observed for resultant PEG113-PBHEm-PSn-Cu micelles, indicating the coordination of His-Cu2+ was favorable to stabilize the cores of micelles. Accordingly, the diameter of blank PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles (Fig. 6) was increased with the increase of PBHE length at pH 7.4 and PEG113-PBHEm-PSn-Cu micelles had a smaller size than the corresponding PEG113-PBHEm-PSn ones. Once in acidic solutions, all micelles with and without Cu2+-coordination presented enlarged diameters compared with those at a neutral condition, which is attributed to the increased solubility of PBHE chains in water. In particular, it was worth noting that different from the neutral condition, there was nearly no difference of diameter between PEG113-PBHEm-PSn and corresponding PEG113-PBHEm-PSn-Cu micelles at pH 5.0, which further confirmed the reversibility of His-Cu2+ coordination. | ||
| Fig. 6 Diameter of PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles at different pH values (n = 3). | ||
DOX loading and in vitro release
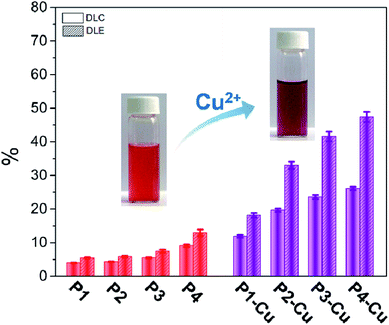
To understand whether the “His-Cu-DOX” can contribute to the improvement of drug loading capacity, the PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles were used as carriers of DOX. For non-coordinated micelles, both their DLC and DLE were lower than 12% (Fig. 7), presenting a very limited drug loading capacity. Compared with this the presence of Cu2+ remarkably improved the capacity of the micelle system. In particular, the DLC and DLE of P4 micelles coordinated with Cu2+ were as high as 26.1% and 47.4%, respectively, which were much higher than those of most carriers based on PS or His cores reported previously.45–48 Interestingly, the color of drug-loaded micelles was red due to the presence of DOX, while the addition of Cu2+ resulted in a change of color to purple, indicating the existence of coordination of DOX.19 Therefore, the increased drug loading capacity was confirmed to be brought by the “His-Cu-DOX” strategy. In addition, with the encapsulation of DOX, an increased diameter was determined for drug-loaded micelles. All their diameters were no higher than 300 nm (Fig. S9†), which could meet the needs of drug carriers. Due to the presence of PBHE with a sufficient electron density, the DOX-loaded P4 micelle samples were evaluated by TEM without staining. Therefore, from the TEM images, the location of PBHE could be distinguished out clearly (Fig. 8). As is reported by micelle systems generally,45,46,49 DOX-loaded P4 micelles presented an approximately sphere-like morphology. Since PBHE was point-like distributed in micelle cores, it was concluded that other than PS with steady hydrophobicity, PBHE also played a role of hydrophobic core. However, it was surprising that an extraordinary morphology was observed for the DOX-loaded P4-Cu micelles, which was attributed to the fact that the encapsulated DOX could not be merely accommodated in the original cores space but in the enlarged ones. Owing to the directivity of coordination bond, the morphology of DOX-loaded P4-Cu micelles became irregular, and PBHE did not stay close any more.A further investigation was conducted to understand the pH-responsive drug release behavior of DOX-loaded PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles. At pH 7.4, PEG113-PBHEm-PSn micelles showed a limited release rate of DOX with cumulative release amount less than 20% within 48 h (Fig. 9a). Once in an acidic solution, the DOX release rate as well as its cumulative release was obviously increased, which was due to the protonation of the imidazole group as well as the increase in micelle diameter (Fig. 6). In order to clearly confirm the size change of micelles in the presence of DOX, diameters of DOX-loaded micelles were evaluated at pH 5.0. During the initial releasing period (6 h for example), both swelling of the PBHE chain induced by the protonation as well as DOX diffusion out from the micelle cores existed, which could make the diameter of the micelles increase. In particular, the diameters of P3 and P4 micelles were determined to be over 400 nm (Fig. S9†). However, with increase in the release time, most loaded DOX was released, resulting in a reverse change of diameter. For instance, the diameter of P4 micelles returned back to about 200 nm, which is close to that of blank micelles (Fig. 6). Other than the environmental pH value, composition of the triblock copolymers had an obvious effect on the release behavior of DOX. With the increase in PBHE content, the release rate as well as the cumulative release of DOX was decreased for PEG113-PBHEm-PSn micelles under both pH conditions. It was worth noting that when Cu2+ was introduced, the cumulative release of all micelle samples was restrained under 10% at pH 7.4 (Fig. 9b). In particular, P4-Cu micelles showed a quite limited cumulative release of DOX, i.e., only 5% after 48 h. What made us more excited was the faster release rate of DOX at pH 5.0 for the Cu2+-coordinated micelles. The cumulative DOX release of all samples was higher than 80% after 48 h at the acidic condition. Moreover, the effect of PBHE amount on the release behavior of DOX was completely different, i.e., an increase in the PBHE amount resulted in a higher cumulative release of DOX at pH 5.0.
 | ||
| Fig. 9 Drug release profiles of DOX-loaded (a) PEG113-PBHEm-PSn and (b) PEG113-PBHEm-PSn-Cu micelles (mean ± standard deviation (SD), n = 3). | ||
To further understand drug release mechanisms of the micelles in the presence or absence of Cu2+ in the acidic environment, first-order and pseudo-second-order kinetics models were applied to analyze their release data. The first-order rate and pseudo-second-order rate equations in linear form50,51 are given as follows:
 | (1) |
 | (2) |
A proposed drug loading and pH-triggered release of PEG113-PBHEm-PSn-Cu micelles is shown in Fig. 10. As designed, DOX can be efficiently encapsulated in micelle cores comprising PS and PBHE via hydrophobic interaction and His-Cu-DOX coordination at neutral conditions, whereas at acidic conditions, a breakup of the coordination among the ligand, metal and drug led to the exhaustive release of DOX out from the micelles.
 | ||
| Fig. 10 Schematic illustration: drug loading process of PEG113-PBHEm-PSn micelles via the His-Cu-DOX coordination architecture and their pH-triggered drug release. | ||
Cytotoxicity and cellular uptake
Since the most efficient drug loading capacity and drug release property were observed for the P4 micelle, it was selected as the carrier of DOX in the cell experiments. Biocompatibility of blank P4 micelles was investigated by the MTT assay. After 48 h incubation, viability of HeLa cells was still more than 95%, indicating that no cytotoxicity was generated from the triblock polymer (Fig. S12†). Compared with this DOX-loaded P4 micelles possessed a proliferation inhibition of HeLa cells. Furthermore, with the introduction of Cu2+, the resultant P4-Cu micelles expressed a higher cytotoxicity with a half maximal inhibitory concentration (IC50) of 1.0 μg DOX equiv. mL−1 (Fig. 11), which was comparable to free DOX. In particular, increasing the drug dosage over 4 μg DOX equiv. L−1 resulted in a viability of HeLa cells less than 15%, presenting an even higher therapeutic effect than the free drug. In order to confirm the cytotoxicity originated from the loaded drug rather than Cu2+, the viability of HeLa cells incubated with Cu2+ at corresponding concentrations was investigated (Fig. S12†). Fortunately, even at the highest concentration of Cu2+ involved in preparation of PEG113-PBHEm-PSn-Cu micelles, the viability of HeLa cells was close to 100%. The results confirmed that the reversible His-Cu-DOX coordination strategy exerted an important effect on the efficient cancer therapy of the pH-responsive drug-loaded micelles. | ||
| Fig. 11 Cytotoxicity of DOX-loaded P4 and P4-Cu micelles and free DOX against HeLa cells after 48 h incubation. | ||
For a superb therapeutic effect, a rapid lysosomal drug release should be achieved; however, compared with a fast entry of free DOX into cell nuclei after cell uptake, transportation efficiency of DOX loaded in the polymeric carriers was generally much lower.52–54 Cell internalization of DOX-loaded P4 and P4-Cu micelles was investigated. As for free DOX, red fluorescence was obviously observed in the whole cell nuclei after only 4 h incubation (Fig. 12). However, very weak DOX fluorescence was determined in cytoplasm around nuclei even after 8 h incubation for DOX-loaded P4 micelles, which can be due to the poor internalization resulting from the stealth effect of PEG shells,55,56 suggesting a poor drug delivery property as well as an inferior lysosomal escape of the non-coordinated micelles. In contrast, because of the high DOX loading content in the presence of Cu2+, an obvious red fluorescence signal could be detected in cytoplasm after just 4 h incubation of HeLa cells with DOX-loaded P4-Cu micelles. With the increase in the incubation time, the fluorescence of DOX in the nucleus became stronger, which indicated that the cleavage of the His-Cu-DOX coordination bond under intracellular acidic environment was very effective to trigger the lysosomal escape of DOX as well as its rapid entry into the nucleus.
Conclusion
In this work, we have developed a novel anticancer drug delivery system based on a pH-responsive ligand–metal-drug coordination interaction strategy. Compared with many other existing systems, our system presented advantages of high drug loading capacity and efficient intracellular drug delivery. First, RAFT polymerization was employed to synthesize a triblock copolymer, PEG113-PBHEm-PSn, which can self-assemble into micelles with diameters less than 200 nm. Incorporation of Cu2+, which can produce coordination interactions with PBHE and DOX, made the size of the micelle decrease to about 100 nm but DOX loading content increased to over 25%. PEG113-PBHEm-PSn micelles showed a very limited premature drug release at pH 7.4, and the presence of Cu2+ could further restrain the drug leakage under 5% within 48 h. However, at an acidic condition, all micelles with and without Cu2+-coordination expressed a quite fast drug release behavior. In particular, the drug release of non-coordinated micelles followed the first-order kinetics model, but the presence of Cu2+ caused the release mechanism of coordinated micelles to comply with a pseudo-second-order kinetics model. This suggests that the cleavage of coordination among the ligand, Cu2+ and DOX played a more important role than diffusion in controlled drug release property. Cell experiments revealed that the Cu2+-coordinated micelles presented an effective suppression of proliferation to HeLa cells which was comparable to free DOX. Moreover, the ligand–metal-drug strategy was beneficial for efficient transportation of DOX into the cell nucleus. The precise design of reversible interaction among ligand, metal and drug achieved desirable physiological circulation stability, loading capacity, intracellular drug delivery and efficient therapeutics, indicating that the micelle carrier was a promising platform for cancer therapy.Experimental section
Materials
Doxorubicin hydrochloride (DOX·HCl, HPLC purity ≥98%) was supplied by Zhongshuo Pharmaceutical Technology Development Co. Ltd. (Beijing, China). Methoxypolyethylene glycol (MPEG113, Mn = 5000) was purchased from Sigma-Aldrich (Shanghai, China). N-(tert-Butoxycarbonyl)-L-histidine (His), N,N-dicyclohexylcarbodiimide (DCC), 2,2′-azobisisobutyronitrile (AIBN) and 4-(dimethylamino)-pyridine (DMAP) were obtained from Aladdin Chemistry Co. Ltd. (Shanghai, China). 2-Hydroxyethyl methacrylate (HEMA) and styrene were purchased from J&K Scientific Ltd. (Beijing, China). 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Beyotime Institute of Biotechnology (Shanghai, China). 2-Dodecylsulfanyl-thiocarbonylsulfanyl-2-methyl propionic acid (DMP) was synthesized according to the literature with a slight modification.57 Other chemicals were purchased from Kelong Chemical Industries Reagent Co. Ltd. (Chengdu, China) and used without further purification.Measurements
1H NMR spectra were recorded on a Bruker Advance 400 spectrometer (Bruker, Germany) using tetramethyl silane (TMS) as an internal reference. Gel permeation chromatography (GPC) was performed on HLC-8320 GPC instrument (Tosoh Co., Japan) with a refractive index detector and a combination of two columns (TSK gel Super AWM-H). The samples were analyzed at 40 °C with DMF as an eluent at a flow rate of 0.6 mL min−1, and the molecular weights were calibrated with poly(methyl methacrylate) standards. High resolution electrospray ionization time-of-flight mass spectrometry (HR ESI-TOF MS) was performed on a Waters Q-Tof Premier mass spectrometer operating in the positive ion electrospray mode. The capillary and sampling cone voltages were set at 2.8 kV and 40 V for positive electrospray mode. The desolvation temperature was set at 150 °C and the source temperature at 90 °C. Infrared spectra were obtained on a Nicolet 6700 Fourier transform infrared spectrophotometer (FT-IR, Thermo Scientific Co., USA) in the 400–4000 cm−1 region with a resolution of 4 cm−1. The average hydrodynamic diameter of aggregates in water were determined at 37 °C by a ZetasizerNano ZS90 (Malvern Instruments, Ltd., U.K.) with a He–Ne laser (633 nm) and 90° collecting optics. UV-Vis absorption spectra were recorded on a Varian Cary® 50 UV-Vis spectrophotometer (Varian Co., USA). The sample was prepared by mixing Cu(NO3)2, DOX·HCl and His together under the stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in phosphate buffer (PB) at different pH values. Fluorescence measurement was carried out on a Varian Cary Eclipse Fluorescence Spectrometer (Varian Co., USA). Raman spectra were acquired using a LabRAM HR spectrometer system (HORIBA JobinYvon, France) with an excitation wavelength of 532 nm generated by an Ar+ laser. Transmission electron microscopy (TEM) was carried out on a Tecnai G2 F20 S-TWIN Transmission Electron Microscope (FEI Co., USA) operated at an acceleration voltage of 200 kV. The sample dispersions were dropped onto carbon-coated copper grids and then air-dried.
1 in phosphate buffer (PB) at different pH values. Fluorescence measurement was carried out on a Varian Cary Eclipse Fluorescence Spectrometer (Varian Co., USA). Raman spectra were acquired using a LabRAM HR spectrometer system (HORIBA JobinYvon, France) with an excitation wavelength of 532 nm generated by an Ar+ laser. Transmission electron microscopy (TEM) was carried out on a Tecnai G2 F20 S-TWIN Transmission Electron Microscope (FEI Co., USA) operated at an acceleration voltage of 200 kV. The sample dispersions were dropped onto carbon-coated copper grids and then air-dried.
Synthesis of Boc-His modified HEMA (Boc-His-EMA)
A mixture of Boc-His (0.89 g, 3.5 mmol), DCC (0.87 g, 4.2 mmol) and a trace of DMAP was dispersed into dry dichloromethane (DCM, 20 mL). The solution of HEMA (0.43 mL, 3.5 mmol) in DCM (10 mL) was then dropped slowly in an ice bath, and then the reaction was carried out for 24 h under room temperature. The resulting mixture was filtered to remove dicyclohexylurea and concentrated under reduced pressure. 1H NMR (D2O, δ, ppm): 1.18–1.32 (s, 9H, (CH3)3–C–NH), 1.81–1.86 (s, 3H, CH3–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 2.77–2.90 (t, H, CH of His), 3.03–3.12 (s, 2H, CH2 of His), 3.73–3.75 4.25–4.29 (t, 4H, 2CH2 of HEMA), 5.61–5.64, 6.05–6.09 (s, H, CH2
C), 2.77–2.90 (t, H, CH of His), 3.03–3.12 (s, 2H, CH2 of His), 3.73–3.75 4.25–4.29 (t, 4H, 2CH2 of HEMA), 5.61–5.64, 6.05–6.09 (s, H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.02–7.08 (s, H, m-H of imidazole), 8.15–8.20 (s, H, o-H of imidazole). HR ESI-TOF MS: m/z 368.01 for [M + H]+; m/z 389.99 for [M + Na]+.
C), 7.02–7.08 (s, H, m-H of imidazole), 8.15–8.20 (s, H, o-H of imidazole). HR ESI-TOF MS: m/z 368.01 for [M + H]+; m/z 389.99 for [M + Na]+.
Synthesis of PEG based chain transfer agent (PEG113-CTA)
A DCM solution (10 mL) of MPEG113 (5.00 g, 1.0 mmol) was added dropwise to a DCM solution (25 mL) containing DCC (0.27 g, 1.3 mmol), DMP (0.44 g, 1.2 mmol) and a trace of DMAP over a period of 30 min. After the reaction mixture was stirred for 24 h at 25 °C, it was filtered to remove dicyclohexylurea and concentrated under reduced pressure. The crude solid product was purified by reprecipitation from DCM into ethyl acetate, yielding 3.8 g light yellow PEG113-CTA powder (71% yield). 1H NMR (CDCl3, δ, ppm): 0.87–0.92 (t, 3H, CH3–C11H22), 1.24–1.36 (s, 20H, –CH2–), 1.71–1.75 (s, 6H, (CH3)2– of DMP), 3.27–3.32 (s, 2H, CH2-CSS), 3.39–3.41 (s, 3H, CH3–O), 3.58–3.79 (t, 452H, CH2CH2O), 4.25–4.29 (t, 2H, CH2–COO).Synthesis of PEG113-PBHEm diblock copolymer
Under a nitrogen atmosphere, prescribed amounts of PEG113-CTA, Boc-His-EMA (1.29 mg, 3.5 mmol), AIBN (5 mg, 0.03 mmol), and DMF (4 mL) were added into a 20 mL glass tube equipped with a magnetic stir bar. After blowing the mixture with nitrogen for 30 min, the tube was sealed and placed in an oil bath thermostat at 75 °C. The polymerization proceeded under stirring for 48 h. The resulting solution was diluted with acetone, and the polymer was precipitated into cold hexane and allowed to stand. After removing the solvent, the block copolymer, PEG113-PHBm, was reprecipitated three times from acetone/hexane and dried under vacuum at room temperature.Synthesis of PEG113-PBHEm-PSn triblock copolymer
PEG113-PHBm-PSn triblock copolymers were synthesized by RAFT polymerization of styrene using PEG113-PHBm as a macro-RAFT agent. Under a nitrogen atmosphere, prescribed amounts of PEG113-PHBm, styrene (0.12 mL, 1.0 mmol), AIBN (5 mg, 0.03 mmol), and DMF (4 mL) were added into a 20 mL glass tube equipped with a magnetic stir bar. After blowing the mixture with nitrogen for 30 min, the tube was sealed and placed at 75 °C for 48 h to allow the polymerization. The resulting solution was diluted with acetone and the product was precipitated with cold hexane. Finally, the triblock copolymer, PEG113-PBHEm-PSn, was reprecipitated three times from acetone/hexane and dried under vacuum at room temperature.Preparation of blank and DOX-loaded PEG113-PBHEn-PSm micelles
PEG113-PBHEm-PSn micelles were prepared by a general dialysis method. The copolymers (10 mg) were first dissolved in 1 mL DMF, and then 10 mL deionized water was added at the rate of one drop per second with vigorous stirring. After being kept for 6 h at room temperature, the turbid solution was transferred into dialysis bags (molecular weight cutoff 3500 Da) and dialyzed for 48 h against PB (pH 7.4, 5 mM). The outer phase was replaced at 8 h intervals with fresh buffer solutions. The Cu2+ coordinated copolymer micelle (PEG113-PBHEm-PSn-Cu) was prepared by mixing the micelle solution of PEG113-PBHEm-PSn with Cu(NO3)2 under the His/Cu2+ stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
For preparation of DOX-loaded micelles, a predetermined amount of DOX in 1 mL DMSO was added slowly into the above-mentioned blank micelle solution under sonication using a probe sonicator. Cu2+ coordination was actuated by further introducing a predetermined amount of Cu2+ in 1 mL DMSO into drug-loaded micelle solution under sonication. The obtained drug-loaded micelle solutions were dialyzed against PB (pH 7.4, 5 mM) for 48 h to remove free DOX and DMSO.
Determination of critical micelle concentration (CMC)
CMC of PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu at pH 7.4 was determined by fluorescence spectroscopy using pyrene as an extrinsic probe. The measurements were carried out at room temperature under an excitation wavelength of 333 nm, and the CMC was determined by taking an inflection point of the polymer concentration at which the relative emission fluorescence intensity ratio measured at 386 nm to 372 nm was varied.Drug loading and in vitro drug release
In order to understand the drug loading capacity of micelles, 0.5 mL of the drug-loaded micelle solution was lyophilized and diluted by 25 mL DMSO to completely release the encapsulated DOX. The obtained solution was analyzed using fluorescence spectroscopy and the characteristic fluorescence emission intensity (λex = 495 nm, λem = 592 nm) of DOX was recorded and compared with a standard curve generated previously. Drug loading content (DLC) and efficiency (DLE) were calculated according to the following equations:| DLC [%] = (W1/W2) × 100 | (3) |
| DLE [%] = (W1/W3) × 100 | (4) |
The drug release experiments were conducted as below: DOX-loaded micelles were added into a dialysis bag (molecular weight cutoff 3500 Da), which was then incubated in 20 mL PB at pH 7.4 or 5.0 at 37 °C in a water bath with a shaking rate at 100 rpm. At predetermined time intervals, 4 mL of incubated solution was taken out and equivalent of fresh PB was replenished. Drug release profiles were determined by measuring the fluorescence absorbance of the release DOX at 592 nm.
Cell culture
Human cervical cancer cells (HeLa) were provided from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 IU mL−1 penicillin and 100 μg mL−1 streptomycin. Cells were grown in a humidified incubator at 37 °C under 5% CO2 atmosphere and harvested with 0.02% EDTA and 0.025% trypsin. The resultant cell suspension was used in the following experiments.Cell viability assays
The relative cytotoxicities of blank micelles, drug loaded micelles and Cu2+ against HeLa cells were evaluated in vitro by MTT assay. The cells were seeded in 96-well plates at a density of 7000 cells per well in 200 μL complete DMEM containing 10% FBS supplemented with 50 U mL−1 penicillin and 50 U mL−1 streptomycin. Cell incubation was performed at 37 °C in the 5% CO2 atmosphere for 24 h. Culture media were then removed, followed by addition of samples at different concentrations. After prescribed hours of incubation, 10 μL of MTT (5 mg mL−1) solution was added to each well and incubation was continued for another 4 h. The medium was removed and 200 μL of DMSO were added into each well to dissolve the formazan by pipetting up and down for several times. The absorbance of each well was measured using a Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., USA) at a test wavelength of 570 nm and a reference wavelength of 630 nm. The cell viability (%) was calculated based on the following equation:
 | (5) |
Cellular uptake
The cellular uptake behavior of DOX-loaded PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles into HeLa cell lines was observed by an inverted fluorescence microscope. The cells were seeded in 6-well plates at a density of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in 2 mL of DMEM containing 10% FBS supplemented with 50 U mL−1 penicillin and 50 U mL−1 streptomycin, and incubated at 37 °C in the 5% CO2 atmosphere for 24 h. The cells were then incubated with DOX-loaded PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles, and free DOX at a final DOX concentration of 8 μg L−1 in DMEM at 37 °C for prescribed hours. After that, the culture medium was removed and cells were washed with PBS thrice, fixed with 4% formaldehyde for 30 min at room temperature, and the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 20 min. Fluorescence microscopy images of cells were obtained by using a Leica DM2500 inverted fluorescence microscope (Leica Microsystems Ltd., Germany).
000 cells per well in 2 mL of DMEM containing 10% FBS supplemented with 50 U mL−1 penicillin and 50 U mL−1 streptomycin, and incubated at 37 °C in the 5% CO2 atmosphere for 24 h. The cells were then incubated with DOX-loaded PEG113-PBHEm-PSn and PEG113-PBHEm-PSn-Cu micelles, and free DOX at a final DOX concentration of 8 μg L−1 in DMEM at 37 °C for prescribed hours. After that, the culture medium was removed and cells were washed with PBS thrice, fixed with 4% formaldehyde for 30 min at room temperature, and the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 20 min. Fluorescence microscopy images of cells were obtained by using a Leica DM2500 inverted fluorescence microscope (Leica Microsystems Ltd., Germany).
Acknowledgements
This work was supported by the Research Fund for the Doctoral Program of Higher Education of China (20130181120067) and the National Natural Science Foundation of China (51403136, 51273123, 51121001 and J1103315). The authors are grateful to Prof. J. L. Yang and Ms L. Yu (State Key Laboratory of Biotherapy and Cancer Center, Sichuan University) for their help for cell experiments.Notes and references
- J. Wang, X. Sun, W. Mao, W. Sun, J. Tang, M. Sui, Y. Shen and Z. Gu, Adv. Mater., 2013, 25, 3670–3676 CrossRef CAS PubMed.
- T. A. Denison and Y. H. Bae, J. Controlled Release, 2012, 164, 187–191 CrossRef CAS PubMed.
- L. Zhu, P. Kate and V. P. Torchilin, ACS Nano, 2012, 6, 3491–3498 CrossRef CAS PubMed.
- M.-H. Xiong, Y. Bao, X.-Z. Yang, Y.-C. Wang, B. Sun and J. Wang, J. Am. Chem. Soc., 2012, 134, 4355–4362 CrossRef CAS PubMed.
- J. S. Lee, T. Groothuis, C. Cusan, D. Mink and J. Feijen, Biomaterials, 2011, 32, 9144–9153 CrossRef CAS PubMed.
- L. Y. Lin, N. S. Lee, J. Zhu, A. M. Nyström, D. J. Pochan, R. B. Dorshow and K. L. Wooley, J. Controlled Release, 2011, 152, 37–48 CrossRef CAS PubMed.
- W. Chen, F. Meng, R. Cheng and Z. Zhong, J. Controlled Release, 2010, 142, 40–46 CrossRef CAS PubMed.
- C. Sanson, C. Schatz, J.-F. Le Meins, A. Soum, J. Thévenot, E. Garanger and S. Lecommandoux, J. Controlled Release, 2010, 147, 428–435 CrossRef CAS PubMed.
- Y. Shi, M. J. van Steenbergen, E. A. Teunissen, L. s. Novo, S. Gradmann, M. Baldus, C. F. van Nostrum and W. E. Hennink, Biomacromolecules, 2013, 14, 1826–1837 CrossRef CAS PubMed.
- F. Shi, J. Ding, C. Xiao, X. Zhuang, C. He, L. Chen and X. Chen, J. Mater. Chem., 2012, 22, 14168–14179 RSC.
- C. Yang, A. B. Ebrahim Attia, J. P. Tan, X. Ke, S. Gao, J. L. Hedrick and Y.-Y. Yang, Biomaterials, 2012, 33, 2971–2979 CrossRef CAS PubMed.
- S. H. Kim, J. P. Tan, F. Nederberg, K. Fukushima, J. Colson, C. Yang, A. Nelson, Y.-Y. Yang and J. L. Hedrick, Biomaterials, 2010, 31, 8063–8071 CrossRef CAS PubMed.
- D.-L. Tang, F. Song, C. Chen, X.-L. Wang and Y.-Z. Wang, Nanotechnology, 2013, 24, 145101–145110 CrossRef PubMed.
- C. Chen, J.-L. Zhou, X. Han, F. Song, X.-L. Wang and Y.-Z. Wang, Nanotechnology, 2014, 25, 255101–255111 CrossRef PubMed.
- R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 CrossRef CAS PubMed.
- Y. Zhou, W. Huang, J. Liu, X. Zhu and D. Yan, Adv. Mater., 2010, 22, 4567–4590 CrossRef CAS PubMed.
- I. Imaz, M. Rubio-Martínez, L. García-Fernández, F. García, D. Ruiz-Molina, J. Hernando, V. Puntes and D. Maspoch, Chem. Commun., 2010, 46, 4737–4739 RSC.
- H. Zheng, Y. Wang and S. Che, J. Phys. Chem. C, 2011, 115, 16803–16813 CAS.
- L. Bai, X.-h. Wang, F. Song, X.-l. Wang and Y.-z. Wang, Chem. Commun., 2014, 51, 93–96 RSC.
- P. Liu, M. Chen, C. Chen, X. Fang, X. Chen and N. Zheng, J. Mater. Chem. B, 2013, 1, 2837–2842 RSC.
- C. Gao, H. Zheng, L. Xing, M. Shu and S. Che, Chem. Mater., 2010, 22, 5437–5444 CrossRef CAS.
- H. Qiao, M. Sun, Z. Su, Y. Xie, M. Chen, L. Zong, Y. Gao, H. Li, J. Qi and Q. Zhao, Biomaterials, 2014, 35, 7157–7171 CrossRef CAS PubMed.
- C. Li, L. Xing and S. Che, Dalton Trans., 2012, 41, 3714–3719 RSC.
- J. M. Benns, J.-S. Choi, R. I. Mahato, J.-S. Park and S. W. Kim, Bioconjugate Chem., 2000, 11, 637–645 CrossRef CAS PubMed.
- Q. Leng, L. Goldgeier, J. Zhu, P. Cambell, N. Ambulos and A. J. Mixson, Drug News Perspect., 2007, 20, 77–86 CAS.
- Y.-F. Zeng, S. Tseng, I. M. Kempson, S.-F. Peng, W.-T. Wu and J.-R. Liu, Biomaterials, 2012, 33, 9239–9245 CrossRef CAS PubMed.
- S. Schmidt, A. Reinecke, F. Wojcik, D. Pussak, L. Hartmann and M. J. Harrington, Biomacromolecules, 2014, 46, 1167–1174 Search PubMed.
- M. M. Pires and J. Chmielewski, J. Am. Chem. Soc., 2009, 131, 2706–2712 CrossRef CAS PubMed.
- K. I. Silva, B. C. Michael, S. J. Geib and S. Saxena, J. Phys. Chem. B, 2014, 118, 8935–8944 CrossRef CAS PubMed.
- J. Tan, X. Rao, J. Yang and Z. Zeng, Macromolecules, 2013, 46, 8441–8448 CrossRef CAS.
- I. Koonar, C. Zhou, M. A. Hillmyer, T. P. Lodge and R. A. Siegel, Langmuir, 2012, 28, 17785–17794 CrossRef CAS PubMed.
- H.-C. Tsai, W.-H. Chang, C.-L. Lo, C.-H. Tsai, C.-H. Chang, T.-W. Ou, T.-C. Yen and G.-H. Hsiue, Biomaterials, 2010, 31, 2293–2301 CrossRef CAS PubMed.
- Y.-C. Chen, L.-C. Liao, P.-L. Lu, C.-L. Lo, H.-C. Tsai, C.-Y. Huang, K.-C. Wei, T.-C. Yen and G.-H. Hsiue, Biomaterials, 2012, 33, 4576–4588 CrossRef CAS PubMed.
- J. T. Wilson, S. Keller, M. J. Manganiello, C. Cheng, C.-C. Lee, C. Opara, A. Convertine and P. S. Stayton, ACS Nano, 2013, 7, 3912–3925 CrossRef CAS PubMed.
- J. Liu, V. Bulmus, D. L. Herlambang, C. Barner-Kowollik, M. H. Stenzel and T. P. Davis, Angew. Chem., Int. Ed., 2007, 46, 3099–3103 CrossRef CAS PubMed.
- S. A. Abraham, K. Edwards, G. Karlsson, S. MacIntosh, L. D. Mayer, C. McKenzie and M. B. Bally, Biochim. Biophys. Acta, Biomembr., 2002, 1565, 41–54 CrossRef CAS.
- A. Srivastava, N. Holten-Andersen, G. D. Stucky and J. H. Waite, Biomacromolecules, 2008, 9, 2873–2880 CrossRef CAS PubMed.
- N. Pekel and O. Güven, Colloid Polym. Sci., 1999, 277, 570–573 CAS.
- H. Takeuchi, Biopolymers, 2003, 72, 305–317 CrossRef CAS PubMed.
- T. Miura, K. Suzuki, N. Kohata and H. Takeuchi, Biochemistry, 2000, 39, 7024–7031 CrossRef CAS PubMed.
- C. Z. Gomez-Castro, A. Vela, L. Quintanar, R. Grande-Aztatzi, T. Mineva and A. Goursot, J. Phys. Chem. B, 2014, 118, 10052–10064 CrossRef CAS PubMed.
- Y. Liu, T. Wang, Z. Li and M. Liu, Chem. Commun., 2013, 49, 4767–4769 RSC.
- J. Shearer, K. E. Rosenkoetter, P. E. Callan and C. Pham, Inorg. Chem., 2011, 50, 1173–1175 CrossRef CAS PubMed.
- H. Wu, L. Zhu and V. P. Torchilin, Biomaterials, 2013, 34, 1213–1222 CrossRef CAS PubMed.
- R. Liu, D. Li, B. He, X. Xu, M. Sheng, Y. Lai, G. Wang and Z. Gu, J. Controlled Release, 2011, 152, 49–56 CrossRef CAS PubMed.
- R. P. Johnson, Y. I. Jeong, E. Choi, C. W. Chung, D. H. Kang, S. O. Oh, H. Suh and I. Kim, Adv. Funct. Mater., 2012, 22, 1058–1068 CrossRef CAS PubMed.
- L. Qiu, Z. Li, M. Qiao, M. Long, M. Wang, X. Zhang, C. Tian and D. Chen, Acta Biomater., 2014, 10, 2024–2035 CrossRef CAS PubMed.
- F. Liu, S. Lin, Z. Zhang, J. Hu, G. Liu, Y. Tu, Y. Yang, H. Zou, Y. Mo and L. Miao, Biomacromolecules, 2014, 15, 968–977 CrossRef CAS PubMed.
- X. Zhang, D. Chen, S. Ba, J. Zhu, J. Zhang, W. Hong, X. Zhao, H. Hu and M. Qiao, Biomacromolecules, 2014, 15, 4032–4045 CrossRef CAS PubMed.
- S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Acta Pol. Pharm., 2010, 67, 217–223 CAS.
- L. Dong, L. Yan, W.-G. Hou and S.-J. Liu, J. Solid State Chem., 2010, 183, 1811–1816 CrossRef CAS PubMed.
- S. Wang, H. Wang, Z. Liu, L. Wang, X. Wang, L. Su and J. Chang, Nanoscale, 2014, 6, 7635–7642 RSC.
- C. Cui, Y.-N. Xue, M. Wu, Y. Zhang, P. Yu, L. Liu, R.-X. Zhuo and S.-W. Huang, Biomaterials, 2013, 34, 3858–3869 CrossRef CAS PubMed.
- J. Cao, X. Xie, A. Lu, B. He, Y. Chen, Z. Gu and X. Luo, Biomaterials, 2014, 35, 4517–4524 CrossRef CAS PubMed.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- F. Zhan, W. Chen, Z. Wang, W. Lu, R. Cheng, C. Deng, F. Meng, H. Liu and Z. Zhong, Biomacromolecules, 2011, 12, 3612–3620 CrossRef CAS PubMed.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra05747g |
| This journal is © The Royal Society of Chemistry 2015 |