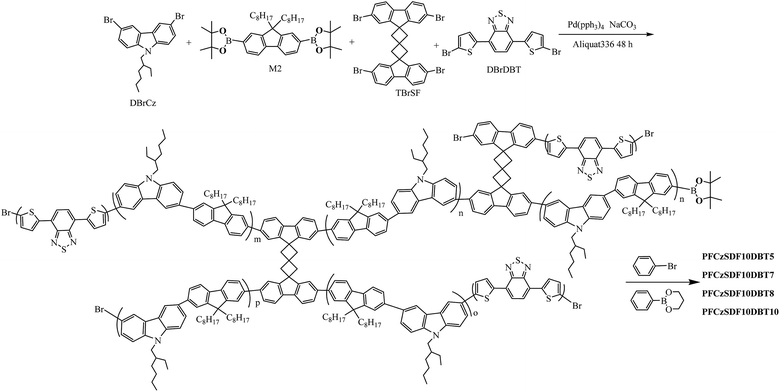
Hyperbranched fluorene-alt-carbazole copolymers with spiro[3.3]heptane-2,6-dispirofluorene as the core and their application in white polymer light-emitting devices†
Yuling Wuab,
Jie Liab,
Wenqing Liangab,
Junli Yangab,
Jing Sunab,
Hua Wangab,
Xuguang Liuc,
Bingshe Xu*ab and
Wei Huangd
aKey Laboratory of Interface Science and Engineering in Advanced Materials, Taiyuan University of Technology, Taiyuan, 030024, China. E-mail: xubs@tyut.edu.cn
bResearch Center of Advanced Materials Science and Technology, Taiyuan University of Technology, Taiyuan, 030024, China
cCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan, 030024, China
dKey Laboratory for Organic Electronics & Information Displays (KLOEID), Institute of Advanced Materials, Nanjing University of Posts and Telecommunications (NUPT), Nanjing, 210046, China
First published on 26th May 2015
Abstract
A series of hyperbranched copolymers with fluorene-alt-carbazole as the branches and three-dimensional-structured spiro[3.3]heptane-2,6-dispirofluorene (SDF) as the core were synthesized by one-pot Suzuki polycondensation. 4,7-Dithienyl-2,1,3-benzothiadiazole (DBT) as the orange-light emitting unit was introduced into the backbones to obtain white-light emission. The thermal, photoluminescent (PL), electrochemical and electroluminescent (EL) properties of the copolymers were investigated. The copolymers show great thermal stabilities by the introduction of a carbazole moiety. Besides, the HOMO energy levels of the copolymers were enhanced and the hole injection was improved because of the hole-transporting ability of the carbazole unit. The hyperbranched structures suppress the interchain interactions efficiently, and help to form amorphous films. The copolymers exhibit efficient EL performance as a result of the hyperbranched structure with the incorporation of the carbazole moiety. A quite low turn-on voltage of 5.3 V, a maximal luminance of 7409.5 cd m−2 and a luminous efficiency of 4.27 cd A−1 were achieved with a CIE coordinate of (0.32, 0.26) for the PFCzSDF10DBT10 (10 mol% of SDF and 0.1 mol% of DBT) device. The hyperbranched framework based on fluorene-alt-carbazole branches and SDF core are attractive candidates for solution-processable white polymer light-emitting device (WPLED) applications.
Introduction
Hyperbranched polymers have recently received considerable interest as light-emitting materials for displays, lighting and other photonic devices.1–5 Firstly, they are easy to be synthesized by one-pot polymerization with comparable properties to dendritic polymers or other well defined polymers.6,7 Secondly, hyperbranched polymers with a three-dimensional structure can prevent the aggregation of polymer chains, making the material form amorphous films with good quality, and increase the glass transition temperature (Tg) of the polymers.4,8,9 Recently, a number of hyperbranched electroluminescent polymers with the three principle colors (red,10 green11,12 and blue13) have been synthesized, and both emission efficiency and thermal stability were effectively improved with respect to their linear analogies.14–16 However, white-light-emitting hyperbranched polymers have rarely been reported. Meanwhile, white polymer light-emitting devices (WPLEDs) have been widely recognized owing to their potential applications in large-area full-color and flexible displays combined with a color filter, backlights and solid lighting sources, and their great advantages such as high stability, low price, as well as easy fabrication process.17–19 A general approach to obtain white-light emission from a single molecule or polymer is to use blue-light emitting and complementary orange-light emitting units.19–21 In our previous research,22 white-light emitting from copolymers based on 9,9-dioctylfluorene and 4,7-dithienyl-2,1,3-benzothiadiazole (DBT) was realized through the incomplete Förster resonance energy transfer (FRET) from the fluorene segment to DBT unit.23 Polyfluorenes (PFs) are shown to be the most promising blue light-emitting materials because of high photoluminescence quantum efficiency, and relatively good chemical and thermal stabilities.24–26 However, it is hard to balance charge injection owing to the large band gap between the HOMO and LUMO energy levels.27 Carbazole is a well-known hole-transporting unit as a result of the electron-donating capabilities associated with its nitrogen atom.13,28 For this reason, to reduce the band gap between the work function of PEDOT:PSS and the HOMO energy levels of the copolymers, and further improve the electroluminescent (EL) performance of the copolymers, carbazole incorporated into the copolymer backbone is a promising strategy.In this paper, hyperbranched copolymers with 3,6-carbazole-alt-2,7-fluorene (PFCz) and DBT branches and spiro[3.3]heptane-2,6-dispirofluorene (SDF) core (10 mol%) were constructed. The three-dimensional-structured SDF exhibits great morphological stability and intense fluorescence,29 and furthermore, its steric hindrance can prevent rotation of the adjacent aryl groups, which reduces close packing and intermolecular interactions between the chromophores in the solid-state.30 In order to obtain white-light emission, the orange light-emitting unit DBT was introduced with different contents from 0.05 mol% to 0.10 mol%. Such a highly branched framework provides a highly efficient white-light electroluminescence.
Experimental section
Materials and characterization
9,9-Dioctylfluorene-2,7-bis(trimethyleneboronate) (M2, 99.5%) was purchased from Synwitech. Tetrahydrofuran (THF) and toluene were distilled using standard procedures. Other solvents were used without further purification unless otherwise specified. All reactions were carried out using Schlenk techniques under dry nitrogen atmosphere. 1H NMR and 13C NMR spectra were measured on a Bruker DRX 600 spectrometer, and chemical shifts were reported in ppm using tetramethylsilane as an internal standard. Elemental analysis (EA) was performed with a Vario EL elemental analyzer. Molecular weights and polydispersities of the copolymers were determined using gel permeation chromatography (GPC) on an HP1100 high performance liquid chromatograph (HPLC) system equipped with a 410 differential refractometer, and a refractive index (RI) detector, with polystyrenes as the standard and THF as the eluent at a flow rate of 1.0 mL min−1 at 30 °C. The UV-visible absorption spectra were determined on a Hitachi U-3900 spectrophotometer and the PL emission spectra were obtained using a Horiba FluoroMax-4 spectrophotometer at room temperature. Thermogravimetric analysis (TGA) of the copolymers was conducted on a Setaram thermogravimetric analyzer at a heating rate of 10 °C min−1 under nitrogen atmosphere. Differential scanning calorimetry (DSC) measurements were performed at both heating and cooling rates of 5 °C min−1 under nitrogen atmosphere, using DSC Q100 V9.4 Build 287 apparatus. Atomic force microscopy (AFM) measurements were performed on an SPA-300HV from Digital Instruments Inc. (Santa Barbara, CA) at a tapping mode. Cyclic voltammetry (CV) measurements were performed on an Autolab/PG STAT302 electrochemical workstation with the thin film of the copolymer on the working electrode in a solution of tetrabutylammonium hexafluorophosphate (Bu4NPF6, 0.1 M) in acetonitrile (CH3CN) at a scanning rate of 50 mV s−1 at room temperature under nitrogen atmosphere. A Pt plate was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode.Device fabrication and characterization
Patterned glass substrates coated with indium tin oxide (ITO) (20 Ω square−1) were cleaned by a surfactant scrub, washed successively with deionized water, acetone and isopropanol in an ultrasonic bath, and then dried at 120 °C in a heating chamber for 8 h. A 40 nm-thick poly(3,4-ethylenedioxythiophene): poly(styrenesulfonic acid) (PEDOT:PSS) hole injection layer was spin-coated on top of ITO and baked at 120 °C for 20 min. Thin films (50 nm thick) of the copolymers as the emitting layer were deposited on top of the PEDOT:PSS layer by spin-coating the chlorobenzene solution of the copolymers, followed by thermal annealing at 110 °C for 20 min. Then an electron-transporting layer of 1,3,5-tris(N-phenylbenzimidazol-2-yl)benzene (TPBi, 35 nm) and LiF (1 nm) and Al (150 nm) as the cathode were deposited by vacuum evaporation under a base pressure of 5 × 10−4 Pa.31 The EL spectra and CIE coordinates were measured with a PR-655 spectra colorimeter. The current–voltage–forward luminance curves were measured using a Keithley 2400 source meter and a calibrated silicon photodiode.Syntheses
Spiro[3.3]heptane-2,6-di-(2′,2′′,7′,7′′-tetrabromospirofluorene) (TBrSDF),32,33 and 4,7-bis(2-bromo-5-thienyl)-2,1,3-benzothiadiazole (DBrDBT)34–36 were synthesized according to the published literature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 5
CH2Cl2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent to give DBrCz as colorless viscous fluid (6.73 g, 15 mmol) in a 77% yield. 1H NMR (600 MHz, CDCl3) δ (ppm): 7.81 (d, J = 1.8 Hz, 2H, Ph), 7.34 (dd, J1 = 1.8 Hz, J2 = 9 Hz, 2H, Ph), 6.93 (d, J = 8.4 Hz, 2H, Ph), 3.65 (t, J = 6.6 Hz, 2H, CH2), 1.78–1.73 (m, 1H, CH), 1.22–1.04 (m, 8H, CH2), 0.77 (t, J = 6.6 Hz, 3H, CH3), 0.73 (t, J = 7.8 Hz, 3H, CH3); 13C NMR (600 MHz, CDCl3) δ (ppm): 142.58, 131.83, 126.24, 126.03, 114.81, 113.52, 50.46, 42.22, 33.86, 31.67, 27.27, 25.94, 16.95, 13.80. Elemental anal. calcd for C20H23Br2N: C 54.94, H 5.30, N 3.20; found: C 54.90, H 5.33, N 3.21.
1 as eluent to give DBrCz as colorless viscous fluid (6.73 g, 15 mmol) in a 77% yield. 1H NMR (600 MHz, CDCl3) δ (ppm): 7.81 (d, J = 1.8 Hz, 2H, Ph), 7.34 (dd, J1 = 1.8 Hz, J2 = 9 Hz, 2H, Ph), 6.93 (d, J = 8.4 Hz, 2H, Ph), 3.65 (t, J = 6.6 Hz, 2H, CH2), 1.78–1.73 (m, 1H, CH), 1.22–1.04 (m, 8H, CH2), 0.77 (t, J = 6.6 Hz, 3H, CH3), 0.73 (t, J = 7.8 Hz, 3H, CH3); 13C NMR (600 MHz, CDCl3) δ (ppm): 142.58, 131.83, 126.24, 126.03, 114.81, 113.52, 50.46, 42.22, 33.86, 31.67, 27.27, 25.94, 16.95, 13.80. Elemental anal. calcd for C20H23Br2N: C 54.94, H 5.30, N 3.20; found: C 54.90, H 5.33, N 3.21.PFCzSDF10DBT5. DBrCz (0.153 g, 0.35 mmol), M2 (0.354 g, 0.55 mmol), TBrSF (0.071 g, 0.1 mmol) and DBrDBT (0.2 ml, 2 × 10−3 mol L−1). Green powder, yield: 62.9%. 1H NMR (CDCl3) δ (ppm): 7.88–7.57 (–ArH–), 6.93–6.89 (–ArH–), 3.41–2.93 (–CH2–), 2.21–1.89 (–C–CH2–), 1.18–0.96 (–CH2–), 0.93–0.55 (–CH3).
PFCzSDF10DBT7. DBrCz (0.153 g, 0.35 mmol), M2 (0.354 g, 0.55 mmol), TBrSF (0.071 g, 0.1 mmol) and DBrDBT (0.28 ml, 2 × 10−3 mol L−1). Light yellow powder, yield: 65.8%. 1H NMR (CDCl3) δ (ppm): 7.89–7.56 (–ArH–), 6.93–6.81 (–ArH–), 3.42–2.93 (–CH2–), 2.21–1.88 (–C–CH2–), 1.19–0.98 (–CH2–), 0.94–0.60 (–CH3).
PFCzSDF10DBT8. DBrCz (0.153 g, 0.35 mmol), M2 (0.354 g, 0.55 mmol), TBrSF (0.071 g, 0.1 mmol) and DBrDBT (0.32 ml, 2 × 10−3 mol L−1). Green powder, yield: 59.3%. 1H NMR (CDCl3) δ (ppm): 8.06–7.42 (–ArH–), 6.94–6.77 (–ArH–), 3.45–3.02 (–CH2–), 2.24–1.87 (–C–CH2–), 1.19–0.95 (–CH2–), 0.94–0.64 (–CH3).
PFCzSDF10DBT10. DBrCz (0.153 g, 0.35 mmol), M2 (0.354 g, 0.55 mmol), TBrSF (0.071 g, 0.1 mmol) and DBrDBT (0.4 ml, 2 × 10−3 mol L−1). Gray powder, yield: 61.4%. 1H NMR (CDCl3) δ (ppm): 7.98–7.48 (–ArH–), 6.93–6.78 (–ArH–), 3.39–3.02 (–CH2–), 2.21–1.75 (–C–CH2–), 1.22–0.88 (–CH2–), 0.86–0.45 (–CH3).
Results and discussion
Synthesis and characterization
As shown in Scheme 1, a series of hyperbranched copolymers based on N-alkyl and 9-C-alkyl substituted 3,6-carbazole-co-2,7-fluorene branches with SDF (10 mol%) as the branch point were synthesized by one-pot Suzuki polycondensation in relatively high yields. To obtain white-light emission, the organe-light-emitting unit DBT was incorporated into the framework with the feed ratios of 0.05 mol%, 0.07 mol%, 0.08 mol% and 0.10 mol%, and the corresponding copolymers are named as PFCzSDF10DBT5, PFCzSDF10DBT7, PFCzSDF10DBT8 and PFCzSDF10DBT10, respectively. The synthetic and structural results of PPFCzSDF10DBT5–PFCzSDF10DBT10 are summarized in Table 1.| Copolymers | nDBrCz | nM2 | nTBrSDF | nDBrDBT | Yield (%) | GPC | |
|---|---|---|---|---|---|---|---|
| Mn | PDI | ||||||
| PFCzSDF10DBT5 | 0.35 | 0.55 | 0.10 | 5 × 10−4 | 62.9 | 9547 | 2.29 |
| PFCzSDF10DBT7 | 0.35 | 0.55 | 0.10 | 7 × 10−4 | 65.8 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 535 |
2.63 |
| PFCzSDF10DBT8 | 0.35 | 0.55 | 0.10 | 8 × 10−4 | 59.3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 854 |
2.43 |
| PFCzSDF10DBT10 | 0.35 | 0.55 | 0.10 | 10 × 10−4 | 61.4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 366 |
1.60 |
The functional groups for Suzuki polycondensation were bromine and trimethyleneboronate. The monomers with bromine group include N-(2-ethylhexyl)-carbazole, the branch point spiro[3.3]heptane-2,6-dispirofluorene (SDF), and 4,7-dithienyl-2,1,3-benzothiadiazole (DBT, ≤0.1 mol%), and the monomers with the trimethyleneboronate group were 9,9-dioctylfluorene. Thus, the fluorene and carbazole monomers distributed alternately in the synthesized polymer branches. Because of the same feed ratios of monomers DBrCz, M2 and TBrSDF for copolymers PPFCzSDF10DBT5–PFCzSDF10DBT10, their 1H NMR spectra were quite similar (Fig. S3, ESI,† the proton signals of DBT have little effect because of its low content), revealing the similar backbone structures of the copolymers. Taking PFCzSDF10DBT7 as an example (Fig. S4, ESI†), the actual ratio of monomers DBrCz, M2 and SDF was calculated by comparing the peak integral intensities of the proton signals of α-CH2 (δ 4.0–4.5), β-CH (δ 1.8–2.2) of N-(2-ethylhexyl)-carbazole, β′-CH2 of 9,9-dioctylfluorene (δ 1.8–2.2), and the spiro[3.3]heptane of SDF (δ 3.0–3.5), and the results (nDBrCz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nM2
nM2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nSDF 0.35
nSDF 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.54
0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) was quite close to the feed ratio of the monomers (0.35
0.1) was quite close to the feed ratio of the monomers (0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.55
0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). The number-average molecular weights (Mn) of the copolymers determined by GPC ranged from 9547 to 10
0.1). The number-average molecular weights (Mn) of the copolymers determined by GPC ranged from 9547 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 with a polydispersity index (PDI) from 1.60 to 2.63. The resulting copolymers are readily soluble in common organic solvents such as CHCl3, THF and toluene.
854 with a polydispersity index (PDI) from 1.60 to 2.63. The resulting copolymers are readily soluble in common organic solvents such as CHCl3, THF and toluene.
Thermal properties
The TGA and DSC data of the copolymers are shown in Fig. 1 and Table 2. The onset decomposition temperatures (Td, measured at a 5% weight loss) range from 400 to 447 °C under nitrogen. The high thermal stability of the copolymers is suggested to benefit from the presence of a large amount of carbazole groups, which are known to exhibit excellent thermal and chemical stabilities.39 The relatively high glass transition temperatures (Tg, inset of Fig. 1) of the copolymers at around 180 °C are found in the DSC curves, which indicates the good morphological stabilities of the copolymers. The high Tgs of the resulting copolymers could be attributed to their rigid hyperbranched structures and the introduction of carbazole unit to the copolymer backbones. | ||
| Fig. 1 TGA and DSC (insert) curves of the copolymers in nitrogen atmosphere with a heating rate of 10 °C min−1 and 5 °C min−1, respectively. | ||
| Copolymer | Td (°C) | Tg (°C) | Dilute solution | Solid film | ||
|---|---|---|---|---|---|---|
| λabs (nm) | λPL (nm) | λabs (nm) | λPL (nm) | |||
| PFCzSDF10DBT5 | 405 | 186 | 364 | 416, 435 | 370 | 418, 439 |
| PFCzSDF10DBT7 | 447 | 186 | 362 | 416, 436 | 367 | 417, 437 |
| PFCzSDF10DBT8 | 400 | 179 | 365 | 416, 436 | 368 | 419, 440 |
| PFCzSDF10DBT10 | 424 | 178 | 371 | 415, 436 | 372 | 419, 439, 603 |
Photophysical properties
The UV-vis absorption of DBT and the PL spectra of fluorene-alt-carbazole copolymer (PFCz) are shown in Fig. S5a.† The absorption of DBT and the emission of PFCz show good spectral overlap, indicating the efficient FRET from the PFCz segment to DBT unit can be expected. The normalized UV-vis absorption and PL spectra of the copolymers in dilute solution are shown in Fig. 2a. The absorption bands at around 365 nm are due to the π–π* transitions of poly(fluorene-alt-carbazole) backbones,27 which are blue-shifted about 20 nm compared with the fluorene-based hyperbranched copolymer.40 This notable hypochromatic shift is attributable to the interruption of the conjugation of the copolymer backbone by the introduction of the 3,6-carbazole linkage. In the PL spectra, the copolymers exhibit emission bands at 416 nm and a slight vibronic shoulder at 436 nm, which can be attributed to the formation of a charge-transfer (CT) state in the poly(fluorene-alt-carbazole) branches. The PL spectra showed about 6 nm blue-shift respect to that of the fluorene-based copolymer because of the decreased conjugated length in these fluorene-alt-carbazole based copolymers.40 No distinct absorption or emission peaks of the DBT unit can be observed in the spectra because of its comparatively low contents in the copolymers, and the FRET is exclusively intrachain in dilute solution.41 | ||
| Fig. 2 UV-vis absorption and PL spectra of the copolymers: (a) in CHCl3 solution (10−5 M) and (b) in solid film. | ||
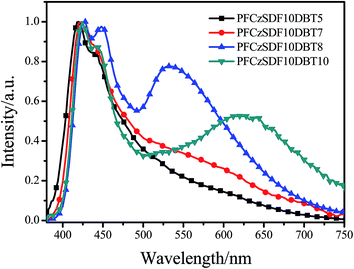
In films, the copolymers exhibit UV-vis absorption bands at around 370 nm owning to the π–π* transitions of the poly(fluorene-alt-carbazole) backbones (Fig. 2b and Table 2). In the PL spectra, the maximum emission bands of copolymers are at about 418 nm, along with a shoulder at around 439 nm, showing no obvious bathochromic shift with respect to those in dilute solution. This result indicates that the hyperbranched molecular structure can prevent the aggregation of the copolymer chains efficiently. For PFCzSDF10DBT10, the emission band of DBT centered at 603 nm can be observed as a result of both intra- and interchain FRET from fluorene-alt-carbazole unit to DBT, which showed a bathochromic shift of 43 nm compared to that of pure DBT as a result of the conjugation effect of the copolymer system.23,41,42
Electrochemical characteristics
The electrochemical behaviors of the resulting copolymers were investigated by cyclic voltammetry (CV), as shown in Fig. 3 and Table 3. The oxidation potentials (Eox) vary slightly from 0.58 to 0.60 V. The HOMO levels of copolymers are calculated according to the empirical formulas EHOMO = −(Eox + 4.5) (eV).41 The LUMO levels are deduced from the HOMO levels and the optical band gaps (Eg) determined from the onset value of the absorption spectrum in film in the long-wavelength direction (Eg = 1240/λedge). The HOMO levels of the hyperbranched copolymers are at about −5.10 eV, which are relatively close to the work function of PEDOT (−5.2 eV) and thus facile hole injection into the emission layer (EML) can be expected.43 On the other hand, the LUMO levels of copolymers (from −2.08 eV to −2.13 eV) are comparatively distant to the work function of LiF/Al (−2.9 eV), indicating that there is a barrier for electron injection. The results demonstrate that the incorporation of the carbazole moiety into the fluorene backbone could effectively increases the hole-transporting ability of the materials, and at the same time, the potential barrier of electron injection is aggrandized. Thus, an electron transport layer (ETL) will be needed in the following PLED structure.| Copolymers | λabs (onset) (nm) | Eg (eV) | Eonset/OX (V) | HOMO (eV) | LUMO (eV) |
|---|---|---|---|---|---|
| PFCzSDF10DBT5 | 416 | 2.98 | 0.60 | −5.11 | −2.13 |
| PFCzSDF10DBT7 | 412 | 3.01 | 0.59 | −5.09 | −2.08 |
| PFCzSDF10DBT8 | 418 | 2.97 | 0.60 | −5.10 | −2.13 |
| PFCzSDF10DBT10 | 419 | 2.96 | 0.58 | −5.08 | −2.12 |
Film morphology
The morphology of the spin-coated films of the copolymers, which is a key factor for the PLED fabrication, was investigated by atomic force microscopy (AFM) at a tapping mode. Fig. 4 shows the AFM images of PPFCzSDF10DBT5–PFCzSDF10DBT10 films prepared by spin-coating chlorobenzene solutions of the copolymers (10−5 M) on the quartz substrates. Generally, all the films show flat and smooth surface without any pinhole defects. The results imply that the three-dimensional structured SDF branch point can help to form homogeneous films with good quality. The uniform amorphous morphology is favorable for PLED fabrication. | ||
| Fig. 4 AFM images (10 × 10 μm) of the copolymer films: (a) PFCzSDF10DBT5, (b) PFCzSDF10DBT7, (c) PFCzSDF10DBT8 and (d) PFCzSDF10DBT10. | ||
Electroluminescent properties
The devices were fabricated with the configuration ITO/PEDOT:PSS (40 nm)/copolymer (50 nm)/TPBi (35 nm)/LiF (1 nm)/Al (150 nm). The relative-energy-level diagram of the devices is shown in Fig. 5. As mentioned above, the incorporation of carbazole unit greatly increases the hole-transporting ability of the materials but leads to a barrier for electron injection into the emission layer. Here 1,3,5-tris(N-phenylbenzimidazol-2-yl)benzene (TPBi) is used as the electron-injection layer to facilitate electron transport.10The electroluminescence spectra of the copolymers are shown in Fig. 6, and the device data are summarized in Table 4. The main peaks at 420 nm with a shoulder at 440 nm are analogous to their PL counterparts. The peaks in the long-wavelength around 610 nm are gradually enhanced with increasing the contents of the orange-light emission unit DBT from PFCzSDF10DBT5 to PFCzSDF10DBT10. White-light emission was obtained with CIE coordinates located near (0.33, 0.33), and the CRI values are around 90 when the contents of DBT were 0.08 mol% and 0.10 mol%. PFCzSDF10DBT8 exhibits cold white light, and PFCzSDF10DBT10 shows warm white light (Fig. 7).
| Copolymer | Vona (V) | Lmaxb (cd m−2) (at the voltage (V)) | CEmax (cd A−1) | LEmax (lm W−1) | CIE (x,y) |
|---|---|---|---|---|---|
| a Turn-on voltage (at 1 cd m−2).b Maximum luminance at applied voltage. | |||||
| PFCzSDF10DBT5 | 5.11 | 7280 (12.6) | 4.38 | 1.64 | (0.22,0.20) |
| PFCzSDF10DBT7 | 5.24 | 7188.5 (13.2) | 4.02 | 1.31 | (0.25,0.24) |
| PFCzSDF10DBT8 | 5.49 | 7266.4 (13.5) | 4.21 | 1.19 | (0.28,0.31) |
| PFCzSDF10DBT10 | 5.34 | 7409.5 (13.5) | 4.27 | 1.45 | (0.32,0.26) |
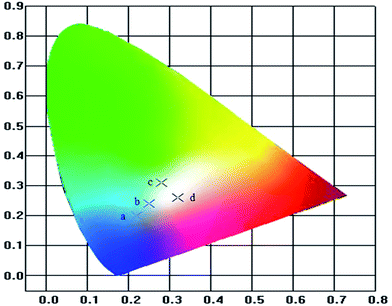 | ||
| Fig. 7 CIE coordinates of the copolymer PLEDs: (a) PFCzSDF10DBT5, (b) PFCzSDF10DBT7, (c) PFCzSDF10DBT8 and (d) PFCzSDF10DBT10. | ||
Taking PFCzSDF10DBT10 as an example, the mechanism of white light emission was investigated from the EL spectra under voltages varying from 8 V to 13 V (Fig. 8a). It is clearly seen that the spectral stabilities at 420 and 440 nm are extremely well and the broad peaks in the long-wavelength around 610 nm are gradually enhanced with increasing the voltages. The growth rate of the intensity of the peak at 610 nm compared to that at 420 nm accelerates as the voltage increases (Fig. 8b). The results reveal that the intra-, interchain FRET from PFCz unit to DBT and the charge trapping of DBT with narrow-band gap happen simultaneously in the electroluminescence process, and the charge trapping is more efficient under higher voltage.23 So the white-light emission of copolymer PFCzSDF10DBT10 was obtained at ⋯V from the blue-light emitting of PFCz segments and the complementary orange-light emitting from DBT through both incomplete intra-, interchain FRET and the charge trapping processes mentioned above.
Fig. 9a shows the current density–voltage–brightness (J–V–L) characteristics of the devices. The devices exhibit moderate low turn-on voltage of 5.1–5.5 V (Table 4), which can be attributed to the small energy barrier between PEDOT:PSS and EML. The maximum luminance (∼7400 cd m−2) and maximum current efficiency (∼4.2 cd A−1) of the devices are quite analogous as a result of the homologous molecular structure of the copolymers. As can be seen from Fig. 9b, the efficiencies decrease quite slowly with increasing current density, suggesting that these hyperbranched copolymers and their devices have good stabilities. Further investigations on the optimization of the device performance are ongoing in our laboratory.
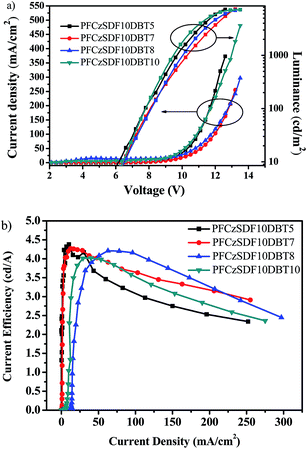 | ||
| Fig. 9 Current–voltage (left) and luminance–voltage (right) curves of copolymer PLEDs (a), and current efficiency–current density characteristics of copolymer PLEDs (b). | ||
Conclusions
In conclusion, a series of hyperbranched 3,6-carbazole-alt-2,7-fluorene copolymers with SDF as the core were prepared by Suzuki polycondensation. The hyperbranched structures suppress the interchain interactions efficiently, and help to form amorphous spin-coating films. The emission bands of the copolymers showed no obvious bathochromic shifts in solid films with respect to those in dilute solution. The FRET efficiency from fluorene-alt-carbazole segments to DBT unit is remained in the hyperbranched systems. With the introduction of carbazole moiety into the backbone, the hyperbranched copolymers show great thermal stability with Tds ranging from 400 to 447 °C and Tgs ranging from 178 to 186 °C, respectively. The HOMO energy levels of the copolymers were close to the work function of PEDOT:PSS because of the carbazole segment, which facilitates hole injection from PEDOT:PSS to EML in the PLEDs. Thus, the hyperbranched copolymers exhibit good EL properties with a low turn-on voltage of about 5 V, the maximum luminance of 7409.5 cd m−2 (at 13.5 V) and maximum current efficiency of 4.38 cd A−1. PFCzSDF10DBT8 and PFCzSDF10DBT10 devices realized white light-emitting with CIE coordinates of (0.28, 0.31) and (0.32, 0.26), respectively. The results indicate that the hyperbranched copolymers using SDF as the core and fluorene-alt-carbazole as the branches could be promising candidates as white-emitting materials with high efficiency.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors are grateful to the “Program for New Century Excellent Talents (NCET) in University” (NCET-13-0927), the International Science & Technology Cooperation Program of China (2012DFR50460), the National Natural Science Foundation of China (21071108, 21101111, 61274056, 61205179, 61307030, 61307029), the Qualified Personnel Foundation of Taiyuan University of Technology (QPFT) (no: tyutrc-201255a), and the Shanxi Provincial Key Innovative Research Team in Science and Technology (2012041011).References and Notes
- T. Guo, L. Yu, B. Zhao, Y. Li, Y. Tao, W. Yang, Q. Hou, H. Wu and Y. Cao, Macromol. Chem. Phys., 2012, 213, 820–828 CrossRef CAS PubMed.
- F. Liu, J.-Q. Liu, R.-R. Liu, X.-Y. Hou, L.-H. Xie, H.-B. Wu, C. Tang, W. Wei, Y. Cao and W. Huang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6451–6462 CrossRef CAS PubMed.
- W. Wu, S. Ye, L. Huang, L. Xiao, Y. Fu, Q. Huang, G. Yu, Y. Liu, J. Qin, Q. Li and Z. Li, J. Mater. Chem., 2012, 22, 6374–6382 RSC.
- A. Pfaff and A. H. E. Müller, Macromolecules, 2011, 44, 1266–1272 CrossRef CAS.
- S. H. Hwang, C. D. Shreiner, C. N. Moorefield and G. R. Newkome, New J. Chem., 2007, 31, 1192–1217 RSC.
- C. He, L. W. Li, W. D. He, W. X. Jiang and C. Wu, Macromolecules, 2011, 44, 6233–6236 CrossRef CAS.
- D. Konkolewicz, C. K. Poon, A. Gray-Weale and S. Perrier, Chem. Commun., 2011, 47, 239–241 RSC.
- N. K. Geitner, B. Wang, R. E. Andorfer, D. A. Ladner, P. C. Ke and F. Ding, Environ. Sci. Technol., 2014, 48, 12868–12875 CrossRef CAS PubMed.
- Y. T. Tsai, C. T. Lai, R. H. Chien, J. L. Hong and A. C. Yeh, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 237–249 CrossRef CAS PubMed.
- T. Guo, R. Guan, J. Zou, J. Liu, L. Ying, W. Yang, H. Wu and Y. Cao, Polym. Chem., 2011, 2, 2193–2203 RSC.
- R. Guan, Y. Xu, L. Ying, W. Yang, H. Wu, Q. Chen and Y. Cao, J. Mater. Chem., 2009, 19, 531–537 RSC.
- J. Liu, L. Yu, C. Zhong, R. He, W. Yang, H. Wu and Y. Cao, RSC Adv., 2012, 2, 689–696 RSC.
- R. Wang, W.-Z. Wang, G.-Z. Yang, T. Liu, J. Yu and Y. Jiang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 790–802 CrossRef CAS PubMed.
- L. Y. Biqing Bao, X. Zhan and L. Wang, Polym. Chem., 2010, 48, 3431–3439 CrossRef PubMed.
- L. R. Tsai and Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4465–4476 CrossRef CAS PubMed.
- L. R. Tsai and Y. Chen, Macromolecules, 2008, 41, 5098–5106 CrossRef CAS.
- M. C. Gather, A. Kohnen and K. Meerholz, Adv. Mater., 2011, 23, 233–248 CrossRef CAS PubMed.
- J. Liu, Y. Cheng, Z. Xie, Y. Geng, L. Wang, X. Jing and F. Wang, Adv. Mater., 2008, 20, 1357–1362 CrossRef CAS PubMed.
- L. Ying, C. L. Ho, H. Wu, Y. Cao and W. Y. Wong, Adv. Mater., 2014, 26, 2459–2473 CrossRef CAS PubMed.
- X. H. Zhu, J. Peng, Y. Cao and J. Roncali, Chem. Soc. Rev., 2011, 40, 3509–3524 RSC.
- B. Zhang, G. Tan, C. S. Lam, B. Yao, C. L. Ho, L. Liu, Z. Xie, W. Y. Wong, J. Ding and L. Wang, Adv. Mater., 2012, 24, 1873–1877 CrossRef CAS PubMed.
- Y. Xu, H. Wang, F. Wei, J. Hou, H. Zhou and B. Xu, Sci. China, Ser. E: Technol. Sci., 2009, 52, 2190–2194 CrossRef CAS PubMed.
- H. Wang, Y. Xu, T. Tsuboi, H. Xu, Y. Wu, Z. Zhang, Y. Miao, Y. Hao, X. Liu, B. Xu and W. Huang, Org. Electron., 2013, 14, 827–838 CrossRef CAS PubMed.
- Z. Ma, S. Lu, Q.-L. Fan, C.-Y. Qing, Y.-Y. Wang, P. Wang and W. Huang, Polymer, 2006, 47, 7382–7390 CrossRef CAS PubMed.
- G. Zeng, W. L. Yu, S. J. Chua and W. Huang, Macromolecules, 2002, 35, 6907–6914 CrossRef CAS.
- Y. H. G. D. Katsis, J. J. Ou, S. W. Culligan, A. Trajkovska, S. H. Chen and L. J. Rothberg, Chem. Mater., 2002, 14, 1332–1339 CrossRef.
- Y. Li, J. Ding, M. Day, Y. Tao, J. Lu and M. D'iorio, Chem. Mater., 2004, 16, 2165–2173 CrossRef CAS.
- J. F. Morin, M. Leclerc, D. Adès and A. Siove, Macromol. Rapid Commun., 2005, 26, 761–778 CrossRef PubMed.
- J. Peng, W. L. Yu, W. Huang and J. Heeger Alan, Adv. Mater., 2000, 12, 828 CrossRef.
- J. Salbeck, F. Weissörtel and J. Bauer, Macromol. Symp., 1998, 125, 121–132 CrossRef CAS PubMed.
- C. Liu, Q. Fu, Y. Zou, C. Yang, D. Ma and J. Qin, Chem. Mater., 2014, 26, 3074–3083 CrossRef CAS.
- S. Yu, H. Lin, Z. Zhao, Z. Wang and P. Lu, Tetrahedron Lett., 2007, 48, 9112–9115 CrossRef CAS PubMed.
- O. P. Y. Moll, T. Le Borgne, P. Thuéry and M. Ephritikhine, Tetrahedron Lett., 2001, 42, 3855–3856 CrossRef CAS.
- W. Shin, M. Y. Jo, D. S. You, Y. S. Jeong, D. Y. Yoon, J.-W. Kang, J. H. Cho, G. D. Lee, S.-S. Hong and J. H. Kim, Synth. Met., 2012, 162, 768–774 CrossRef CAS PubMed.
- Z. Wang, P. Lu, S. Xue, C. Gu, Y. Lv, Q. Zhu, H. Wang and Y. Ma, Dyes Pigm., 2011, 91, 356–363 CrossRef CAS PubMed.
- B. Liu, A. Najari, C. Pan, M. Leclerc, D. Xiao and Y. Zou, Macromol. Rapid Commun., 2010, 31, 391–398 CrossRef CAS PubMed.
- J. Kim, S. H. Kim, J. Kim, I. Kim, Y. Jin, J. H. Kim, H. Y. Woo, K. Lee and H. Suh, Macromol. Res., 2011, 19, 589–598 CrossRef CAS.
- H. Wang, J. T. Ryu and Y. Kwon, J. Appl. Polym. Sci., 2011, 119, 377–386 CrossRef CAS PubMed.
- T. Sudyoadsuk, P. Moonsin, N. Prachumrak, S. Namuangruk, S. Jungsuttiwong, T. Keawin and V. Promarak, Polym. Chem., 2014, 5, 3982–3993 RSC.
- For the fluorene-based hyperbranched copolymer with SDF branch point, the absorption peak was at about 384 nm, and the emission peaks were at 420, 442, and a shoulder peak at 468 nm. This is our another work, and it will be published elsewhere.
- J. Yang, C. Y. Jiang, Y. Zhang, R. Q. Yang, W. Yang, Q. Hou and Y. Cao, Macromolecules, 2004, 37, 1211–1218 CrossRef CAS.
- Z. Wang, P. Lu, S. Xue, C. Gu, Y. Lv, Q. Zhu, H. Wang and Y. Ma, Dyes Pigm., 2011, 91, 356–363 CrossRef CAS PubMed.
- M. Zhu, Y. Li, J. Miao, B. Jiang, C. Yang, H. Wu, J. Qin and Y. Cao, Org. Electron., 2014, 15, 1598–1606 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR characterization of DBrCz and 1H NMR characterization of the copolymers and PFCzSDF10DBT7. UV-vis absorption of DBT and PL spectra of PFCz in CHCl3 solution (10−5 mol L−1), and PL spectra of DBT in CHCl3 solution (10−5 mol L−1). See DOI: 10.1039/c5ra05713b |
| This journal is © The Royal Society of Chemistry 2015 |