Acetaldehyde in asymmetric organocatalytic transformations
Manjeet Kumar†
a,
Arvind Kumar†a,
Masood Ahmad Rizvi*b and
Bhahwal Ali Shah*a
aAcademy of Scientific and Innovative Research, Natural Product Microbes, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu-Tawi, 180001, India. E-mail: bashah@iiim.res.in; masoodku2@gmail.com
bDepartment of Chemistry, University of Kashmir, Srinagar, India
First published on 17th June 2015
Abstract
The role of acetaldehyde as a nucleophile in various asymmetric C–C bond forming transformations is presented, with consideration given not only to the optimization of reaction parameters relevant to product formation, polymerization, by-products, purification, chirality and instability of the final products, but also to the application of the final products in the synthesis of bioactive molecules. This review is organized according to the use of acetaldehyde in different organocatalytic reactions covering the most recent reports. In the last section indirect sources of acetaldehyde are discussed from the perspective of difficult handling and its requirement of slow addition as well as being freshly distilled in some of the transformations, which is one of the most critical issues in the late exploitation of acetaldehyde as a nucleophile in synthetic chemistry.
1. Introduction
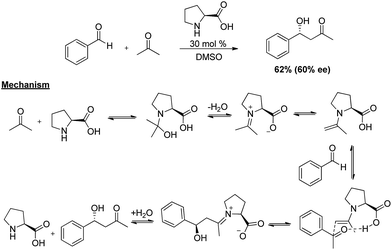
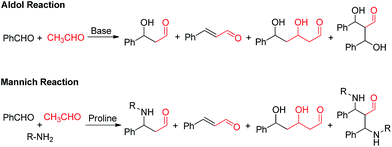
Asymmetric C–C bond formation is one of the most important and challenging key transformations in organic chemistry,1,2 providing access to a wide variety of building blocks used in chiral drug development.3 The importance of enantioselective C–C bond formation can also be inferred from the large amount of research that has been performed on the reactions like asymmetric aldol,4 Diels–Alder,5 Michael,6 allylic alkylation,7 Mannich,8 and Baylis–Hillman reactions.9 Previously, these transformations were generally achieved by either of two catalytic approaches i.e., metal catalysts employing mainly transition metals or biocatalysts wherein enzymes were used for their efficiency and selectivity.10 However in the last decade a third front, the field of organocatalysis has grown with a breath-taking pace and has emerged as powerful tool to construct enantiorich compounds.11 This can be easily visualised from the large number of research papers published in last decade.12 Although Hajos et al.13 and Wiechert et al.14 had independently reported the first highly enantioselective proline catalysed intramolecular aldol reaction in the early 1970's; organocatalysis was not considered as a possible alternative to two main classes of established asymmetric catalysis (metal catalysis and biocatalysis). It was in 2000 when Barbas and co-workers15 employed proline in enantioselective intermolecular cross aldol reaction (Scheme 1), marking the explosive growth of low molecular-weight enamine/iminium-ion organocatalysis. The reaction involves asymmetric enamine catalysis in which carbonyl compounds form reactive enamine intermediate with L-proline catalyst, increasing the feasibility of reacting with electrophiles.2. Challenges associated with the use of acetaldehyde
The recent advances in the design and development of organocatalysts has not only provided the easy and more sustainable alternate to metal complexes in organic synthesis but also offers a greater scope in terms of coupling partners (nucleophiles & electrophiles). Various nucleophiles have made their way into the world of chemical diversity through these synthetic methodologies. Despite all advances the simplest of all nucleophiles, acetaldehyde, was not much used until recently16 due to various reasons such as (a) it's low boiling point (21 °C), generally involves sensitive reaction protocols; (b) its ability to act as nucleophile as well as electrophile, give rise to uncontrolled cascade reactions like polyaldolization, double Mannich etc.; (c) its tendency to polymerize at room temperature requires freshly distilled acetaldehyde for product formation in good yields; (d) dehydration of the final product enables Michael type additions; (e) its high reactivity results in self-aldol condensation prior to cross coupling; (f) undergo Tishchenko-type processes; (g) the small steric difference between the methyl and hydrogen atom suffers with low relative control, resulting in decreased enantioselectivity (Scheme 2).3. Acetaldehyde as a nucleophile
3.1. Use of acetaldehyde in aldol reactions
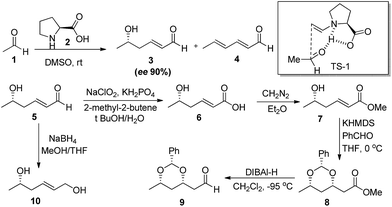
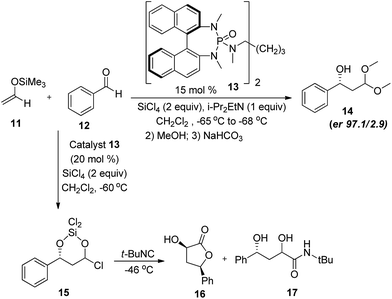
Aldol reaction is one of the most studied reactions for C–C bond formation in the history of synthetic chemistry.17 This may be attributed to the versatility offered by this reaction in terms of coupling partners with the ease of use.18 Owing to the difficult handling, acetaldehyde was not directly used as a nucleophile in organocatalysed C–C bond forming reactions until 2002, when Barbas et al.19 reported the first time direct use of acetaldehyde (1) in self aldol reaction. In the initial investigation, the proline (2) catalysed self aldol reaction in DMSO at room temperature lead to the formation of two products, i.e. (+)-(5S)-hydroxy-(2E)-hexenal (3) and 2,4-hexadienal (4), where dehydrated product (3) was the major one. Standardisation of the reaction condition i.e. in THF at 0 °C, afforded the desired product in high enantiomeric excess (90%) however the yield was low (10%). Proposed mechanistic origin of stereoselectivity involves the re-facial attack of enamine on carbonyl group of acetaldehyde in transition state (TS-1) which further undergoes Mannich type condensation to give the product. Authors also addressed the importance of products by converting them into various important building blocks commonly encountered in the synthesis of macrolide antibiotics such as grahamimycin A and A1, carbomycin B and platenomycin A20(Scheme 3).After these initial efforts, other reports followed where Denmark and Bui21 used trialkylsilylenol ether of acetaldehyde in chiral phosphoramide catalysed enantioselective aldol addition with aromatic aldehydes. The reaction of trimethylsilyl enol ether of acetaldehyde (11) and benzaldehyde (12) in the presence of 15 mol% of chiral dimeric phosphoramide catalyst (13), SiCl4 (2 equiv.) and i-Pr2EtN (1 equiv.) gave the desired aldol addition in 80% yield and 97.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 enantiomeric ratio. Since the product was unstable thereby reaction was quenched with methanol which resulted in dimethylacetal protected aldol product (14). Furthermore, reaction proceeds through the formation of chlorohydrin intermediate (15) which in the presence of tert-butyl isocyanide had been converted to lactone (16, d.r. 9/1) and β-hydroxy amide (17) in a single step (Scheme 4).
2.9 enantiomeric ratio. Since the product was unstable thereby reaction was quenched with methanol which resulted in dimethylacetal protected aldol product (14). Furthermore, reaction proceeds through the formation of chlorohydrin intermediate (15) which in the presence of tert-butyl isocyanide had been converted to lactone (16, d.r. 9/1) and β-hydroxy amide (17) in a single step (Scheme 4).
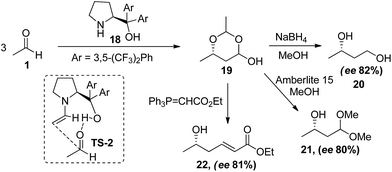
Although proline has been used in the self aldol reaction of acetaldehyde19 but reaction afforded the product in poor yields (12%). To overcome the issues related to yields, Hayashi and co-workers22 successfully attempted the use of trifluoromethyl substituted diaryl prolinol (18) to give product (19) in good yields and high enantioselectivities. The reaction was screened in different solvents where the best results were obtained in N-methyl pyrrolidone affording the product in 56% yield and 82% enantioselectivity. Furthermore, the product obtained was in situ acetal protected, which had been converted to the different important building blocks (21, 22) in same pot (Scheme 5). In the proposed transition state (TS-2), diarylprolinol forms anti-enamine complex with one equivalent of acetaldehyde and directing the approach towards (S)-phase of other electrophilic acetaldehyde through hydrogen bonding between acidic proton of catalyst and carbonyl oxygen of acetaldehyde.
 | ||
| Scheme 5 Asymmetric, catalytic and direct self-aldol reaction of acetaldehyde catalyzed by diaryl prolinol. | ||
3.2. Mannich reaction
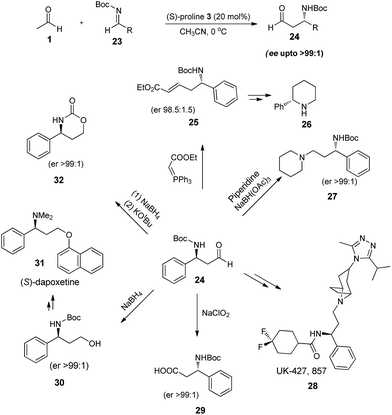
Chiral β-amino carbonyl compounds are always considered as privileged motif in medicinal chemistry, owing to their high abundance in natural products as well as in drug like molecules. They are largely accessed through Mannich reaction,23 which is arguably the most simple, economical approach to C–C bond formation in nitrogen containing compounds and has flourished over the years due to the ubiquitous potential of this multicomponent reaction to generate diversity by means of substrate combinations. However acetaldehyde was not used as a substrate in Mannich reaction until 2008, when List et al.,24 for the first time reported the direct use of acetaldehyde in (S)-proline catalysed Mannich reaction. They came up with the observation that all the side reactions (Scheme 2) can be suppressed if higher excess of acetaldehyde (5–10 equivalents) is employed. Thus (S)-proline (20 mol%) catalysed three component reaction of aromatic as well as aliphatic N-Boc-imines (23) with acetaldehyde (10 equiv.) in acetonitrile at 0 °C gave the β-amino aldehydes (24) in moderate yields (23–58%) and high enantioselectivity ratio upto >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The importance of Mannich product was also highlighted by using it as a chiral building block in the synthesis of variety of bioactive molecules e.g., (24) can readily undergo Wittig reaction to give δ-amino-α,β-unsaturated ester (25), a synthetic intermediate of substituted piperidines (26) which are highly abundant in bio-active molecules.25 Furthermore, the reductive amination of aldehyde also resulted in the formation of piperidine derivatives (27) which found its application in the synthesis of UK-427, 857 (28), a recently approved CCR5 inhibitor for the HIV treatment.26 To broaden the further application of asymmetric Mannich product, author reduced it to amino alcohol (30), which is a known intermediate in the synthesis (S)-dapoxetine (31), a selective serotonin re-uptake inhibitor.27 Also in situ cyclisation of reduced alcohol lead to the formation of oxazinone (32), an important heterocyclic synthetic intermediate.28 In addition, enantiopure β3-amino acids29 (29) can also be synthesized by oxidation of Mannich product in the presence of sodium hypochlorite (Scheme 6).
1. The importance of Mannich product was also highlighted by using it as a chiral building block in the synthesis of variety of bioactive molecules e.g., (24) can readily undergo Wittig reaction to give δ-amino-α,β-unsaturated ester (25), a synthetic intermediate of substituted piperidines (26) which are highly abundant in bio-active molecules.25 Furthermore, the reductive amination of aldehyde also resulted in the formation of piperidine derivatives (27) which found its application in the synthesis of UK-427, 857 (28), a recently approved CCR5 inhibitor for the HIV treatment.26 To broaden the further application of asymmetric Mannich product, author reduced it to amino alcohol (30), which is a known intermediate in the synthesis (S)-dapoxetine (31), a selective serotonin re-uptake inhibitor.27 Also in situ cyclisation of reduced alcohol lead to the formation of oxazinone (32), an important heterocyclic synthetic intermediate.28 In addition, enantiopure β3-amino acids29 (29) can also be synthesized by oxidation of Mannich product in the presence of sodium hypochlorite (Scheme 6).
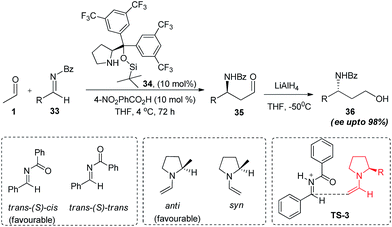
In the same year Hayashi and co-workers reported the trifluoromethyl-substituted diaryl prolinolsilyl ether catalysed Mannich reaction of acetaldehyde with N-benzoyl imine (33).30 It was however found that reaction works only in the presence of weak acidic additive like benzoic acids while strong acid like TsOH results in the decomposition of imine. The reaction in the presence of additive as p-nitrobenzoic acid in anhydrous THF affords the product (35) in good yields and excellent enantioselectivities (up to 98%). In this paper authors also presented the mechanistic insights of the reaction through quantum energy calculations where they found that trans N–C(O) bond having (S)-cis-geometry of imine and anti-conformation of enamine are the low energy transition states. According to the energy calculations, attack of trans-enamine at si-face of protonated imine is a highly exothermic process having large negative energy (−38.82, −31.12 kcal mol−1 in gas phase and THF phase respectively, calculated through B3LYP theory, TS-3). This suggests that formation of enamine from acetaldehyde and diarylprolinolsilyl ether involving several steps (attack of N-atom of catalyst at the carbonyl centre of acetaldehyde, proton transfer, and dehydration) is the rate determining step. After which the formation C–C bond formation take place with almost no activation barrier. This was further confirmed by Gan and co-workers31 in the detailed mechanistic studies of reaction through density functional theory calculations (Scheme 7).
 | ||
| Scheme 7 Acetaldehyde in trifluoromethyl-substituted diaryl prolinolsilyl ether catalysed Mannich reaction. | ||
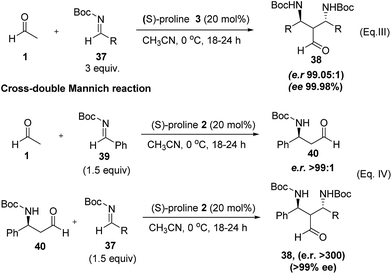
List et al.,32 also reported the use of acetaldehyde in double Mannich reaction where one equivalent of acetaldehyde was treated with three equivalents of N-Boc-imines (37, 3 equiv.) in the presence of (S)-proline in acetonitrile at 0 °C. The reaction was found to give double Mannich product (38) with remarkably high enantioselectivities with both (S) and (R)-proline; however, the products with aliphatic aldehyde were obtained in low yields (Scheme 8, eqn (III)). Methodology was further extended to cross-Mannich reaction with two different imines wherein, initial mono-addition products were firstly isolated and then subjected to a second reaction with different aromatic N-Boc imines (Scheme 8, eqn (IV)).
In 2009, Maruoka and co-workers33 reported the use of acetaldehyde in direct asymmetric Mannich reaction of N-Boc-protected imines (41). The reaction was performed with axially chiral bifunctional amino sulphonamide catalyst (42), where the author proposed that highly acidic triflamide group played a crucial role inactivating the electrophile with an added advantage i.e. dibenzylic secondary amine are less nucleophilic than pyrrolidine type catalysts thus reduces the possibility of the side product formation in Mannich reaction. Initially reaction was done in various solvents later on they found the best results in solvent free conditions at 0 °C. Reaction was applicable to both aromatic as well as aliphatic imines without any significant change in either of yields or enantioselectivity. Also, author attempted the reaction with different α-substituted aldehydes which afford anti-Mannich adducts with high enantioselectivities (Scheme 9).
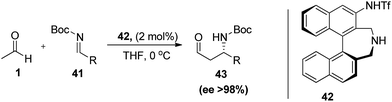 | ||
| Scheme 9 Chiral amino sulfonamide as an efficient organocatalyst for direct asymmetric Mannich reactions of N-Boc-protected imines. | ||
3.3. Cross-aldol reaction
The high abundance of asymmetric β-hydroxyl carbonyl moiety in natural products has always excited the research community for development of their new synthetic methods. Over the years cross-aldol reaction has flourished as the simplest and most economical way to introduce this functionality in stereochemical fashion. In 2008, Hayashi et al.34 first time exploited the nucleophilicity of acetaldehyde in organo-catalysed cross-aldol reaction wherein they employed various aromatic as well as aliphatic aldehydes as eletrophiles in the presence of bistrifluoromethyl substituted diarylprolinol (18). In an initial attempt they employed L-proline (2) as a catalyst in reaction between 2-chlorbenzaldehyde (44) and acetaldehyde, but instead of giving any cross-aldol product, reaction underwent self-condensation of acetaldehyde and gave crotonaldehyde (45) as a major product (Scheme 10, eqn (V)). Screening different proline derivatives author found that trifluoromethyl-substituted diarylprolinol catalyst (18) is suitable. Thus, on employment of (18), the reaction of acetaldehyde with variety of aromatic aldehydes gave cross-aldol product (48) in good yields (52–92 %) and high enantioselectivities (80–99%) (Scheme 10, eqn VI). As the reaction in the presence of diarylprolinol based silyl ether affords the product in poor yields suggesting the role of hydrogen bonding in activating carbonyl group of aromatic aldehyde for reaction (TS-4). Since, the product obtained was not stable and undergo dehydration when tried to isolate, so the cross-aldol product was further reduced to diol (49) in the presence of sodium borohydride in methanol at 0 °C.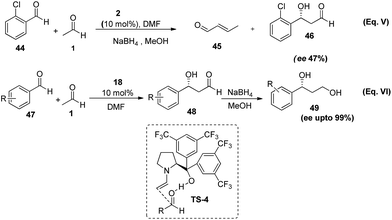 | ||
| Scheme 10 Acetaldehyde in trifluoromethyl substituted diarylprolinol catalyzed cross Aldol reaction. | ||
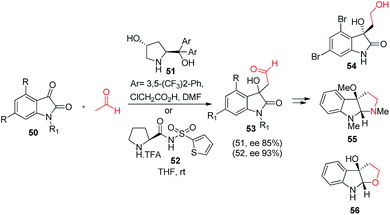
Isatins have recently gained an increased interest due to its privileged scaffold, a large abundance in biologically potent molecules and greatly its synthetic versatility led to the generation of a large number of structurally diverse organic compounds. After the successful implication of acetaldehyde in cross-aldol reaction with various aromatic aldehydes, Nakamura et al.35 and Hayashi et al.36 independently reported the reaction of acetaldehyde with isatin in the presence of N-(heteroarenesulfonyl)prolinamides (52) and 4-hydroxydiarylprolinol (51) organocatalysts respectively. In both cases reactions afforded the product in good yields with high enantioselectivities. The importance of obtained product was also shown in the synthesis of 3-hydroxyindole alkaloids (Scheme 11). The reactions involve isatin aldol product as a key intermediate in the synthesis of ent-convolutamydine E (54) and CPC-1 (55) and a half fragment of madindoline A and B (56).
Zhao and coworkers37 also reported a similar reaction of acetaldehyde and isatin where cinchona primary amine (58) and benzoic acid were used as catalysts and co-catalyst respectively. Standardization studies shows that water has a significant effect on reactivity of the reaction i.e., 3 equiv. of water in THF solvent decreases the reaction time from 48 to 15 h with no change in yield however there is slight increase in enantioselectivity from 87–93%. Proposed mechanism indicates the multiple roles of benzoic acid like it accelerates the enamine formation between primary amine moiety of catalyst and acetaldehyde, simultaneously, it also protonates the N atom of quinicilidine ring which forms hydrogen bond with isatin carbonyl groups. In the transition state enamine approaches the re-face of isatin ketone group resulting in major S-enantiomer (TS-5, TS-6) (Scheme 12).
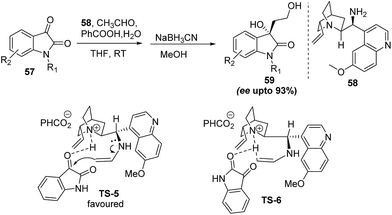 | ||
| Scheme 12 Acetaldehyde in trifluoromethyl substituted diarylprolinol catalyzed cross aldol reaction. | ||
Other report by Yuan and coworkers,38 where 4-hydroxy diarylprolinol (51) is used as a catalyst in the reaction between acetaldehyde and isatin. Reaction without any co-catalyst affords the products in high yields and good enantioselectivities. To demonstrate the synthetic utility of reaction method, author successfully attempted the synthesis of various biologically active compounds like (R)-convolutamydines B (62) and E (63), (−)-donaxaridine (64) and (R)-chimonamidine (65) (Scheme 13).
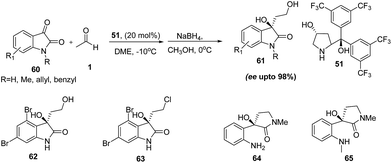 | ||
| Scheme 13 4-Hydroxy diarylprolinol is used as a catalyst in the reaction between acetaldehyde and isatin. | ||
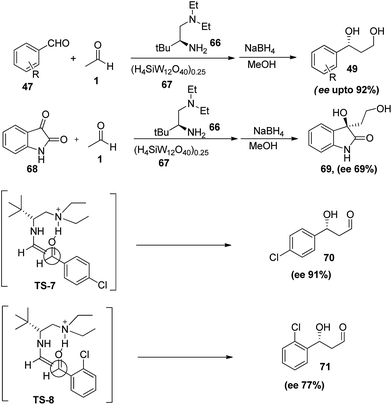
In the last decade number of chiral primary amine-based organocatalysts had been developed for various carbon–carbon bond forming reactions.39 Cheng et al.,40 had successfully applied primary amines as the organocatalysts in aldol reaction of acetaldehyde with aromatic aldehyde and isatin derivatives. The reaction of L-tert-leucine derivative organocatalyst (66) in conjunction with (H4SiW12O40)0.25 (67) affords the product in excellent yields (34–99%) and high enantioselectivities (up to 92%) with range of aromatic aldehydes. Furthermore primary amines were also employed in aldol reaction of acetaldehyde with isatin derivatives where desired product was obtained in excellent yields and moderate to good enantioselectivites (Scheme 14). Proposed mechanism involves hydrogen bonding between protonated amino group and carbonyl group of acceptor aldehyde which plays a significant role in governing the stereochemical outcome. In addition, the bulky tert-butyl group and the substituents on tertiary amine also favour the si-face attack of enamine to the acceptor aldehyde. Hypothesis was further confirmed by the results obtained in case of o- and p-chloro substituted benzaldehydes where o-chlorobenzaldeyde gave the product with low enantioselectivity because of steric interference between ortho substituent and tertiary amino group (TS-7) while in case of para no such interaction take place, thus desired product in high enantioselectivity (TS-8).
Qiao and co-workers developed a new dioctylamino group containing diarylprolinol-based catalyst (72) for cross aldol reaction of acetaldehyde in aqueous medium.41 The reaction in the presence of catalyst (72, 5 mol%), ionic liquid supported benzoic acid (73, 10 mol%) as co-catalyst, afforded product in good yields upto 97% in brine water. Even the reaction at low catalyst loading of 5 mol% affords high enantioselectivities upto 92% in brine solution with a range of aromatic aldehydes (Scheme 15).
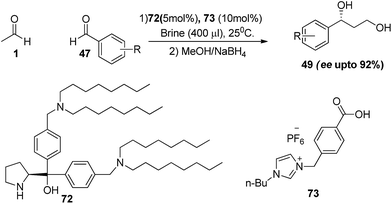 | ||
| Scheme 15 Dioctylamino group containing diarylprolinol-based catalysed cross aldol reaction of acetaldehyde. | ||
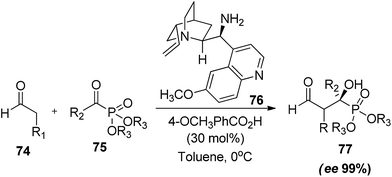
Quinine and 4-methoxybenzoic acid mediated cross-aldol reaction of acetaldehyde with α-ketophosphonate afforded a series of β-formyl-α-hydroxyphosphonates in good yields and moderate to good enantioselectivites.42 In an Initial attempt reaction was tried between propanal (74) and α-ketophosphonate (75) which undergo no product formation. The accepted reason was the unfavourable interaction between enamine methyl group and the bulky phosphonate group in the transition state. Thus, the reaction was further tried with acetaldehyde using proline and prolinamide as catalysts. As expected reaction undergo the product formation in significant quantity (50% and 59% respectively), but after a prolonged reaction time (7 days). Various catalysts had been screened where quinine derivative (76) were found to be suitable for reaction conditions and affording the product (77) in 75% yield and 93% ee in the presence of 4-methoxybenzoic acid as additive and toluene as solvent (Scheme 16). Both the electron donating and electron withdrawing substituents on the phenyl ring of benzoyl-phosphonates worked well under the standard reaction conditions. Furthermore the obtained products were screened for their anticancer potential where they were found to suppress the proliferation of human and murine tumor cells, while are mild against immortalized cells (HFF).
3.4. Michael reaction
Acetaldehyde also found its application in one of the vastly studied organo-catalysed reaction i.e. conjugate addition of nucleophiles to the β-position of α,β-unsaturated carbonyl compounds, known as Michael reaction.43 List and Hayashi and their co-workers independently reported the use of acetaldehyde in diarylprolinol (79) catalyzed Michael reaction of various substituted nitro styrenes (Scheme 17).44,45 Further List et al., have demonstrated the synthetic potential of obtained Michael product in pharmaceutically important compounds such as (R)-pregabalin (81)46 (S)-baclofen (82),47 (S)-rolipram (83)48 and 3-monosubstituted pyrrolidines (84).49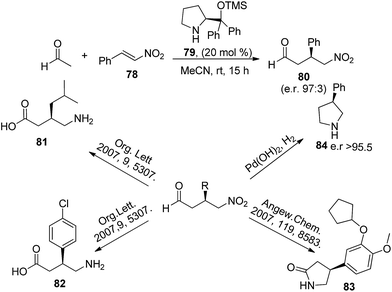 | ||
| Scheme 17 Acetaldehyde in trifluoromethyl substituted diarylprolinol silyl ether catalysed Michael reaction. | ||
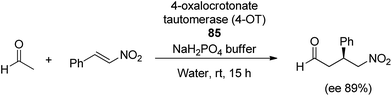
Inspired by the versatility of proline and its derivatives, Zandvoort and co-workers50 designed new biocatalysts (85) by combining organocatalyst with biocatalyst for Michael-type addition reaction of acetaldehyde with substituted β-nitrostyrenes. Reaction involved the use of 4-oxalocrotonate tautomerase (0.7 mol%) in water, afforded the product in moderate yield and high enantioselectivity up to 89% (Scheme 18).
3.5. Miscellaneous reactions
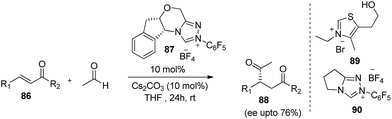
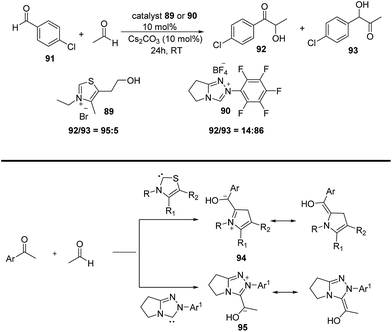
After remarkable success in aldol, Mannich, Michael reaction, acetaldehyde was further used up in N-heterocyclic carbene-catalysed intermolecular Stetter reactions i.e.1,4-addition of aldehyde to an α,β-unsaturated compound. Yang et al.,51 used acetaldehyde for first time as source of acyl anion which further engaged up in the reaction with Michael acceptors. Initially the reaction was screened with thiazolium (89) and triazolium precatalyst (90) with Cs2CO3 which provides the desired product in good yields but in racemic fashion. Further to achieve asymmetric product author employ other NHC catalysts where they found chiral cis-2-aminoindanol-derived triazolium salt (87) having N-penta fluorophenyl substituent gave the better results with yield upto 85% and enantioselectivity upto 76%.Same group employed acetaldehyde in regioselective acyloin condensation wherein the reaction of acetaldehyde with electrophilic aromatic aldehydes in the presence of (89, 90) afforded the desired product52 (Scheme 22). According to proposed mechanism, in case of thiazolium catalyst the nucleophilic attack of NHC catalyst occurs on aromatic aldehydes rather than acetaldehyde, affords the most resonance-stabilized Breslow intermediate (94). In the next step nucleophilic carbene thus formed reacts with acetaldehyde to give the thermodynamically stable product (92). In contrast, triazolium catalyst (90) involves the first nucleophilic attack on acetaldehyde 95 rather than aromatic aldehydes because of steric requirements and thus leads to the formation of product (93). In addition, authors had also described the effect of electronic properties of aromatic aldehydes on regioselective outcome. Thiazolium catalyst (89), gave better regioselectivity (higher 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) for electron-withdrawing substituents on the aromatic aldehydes while triazolium catalyst (90), the regioselectivity is better (higher 4
4 ratio) for electron-withdrawing substituents on the aromatic aldehydes while triazolium catalyst (90), the regioselectivity is better (higher 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) for electron-donating substituents on the aromatic aldehydes.
3 ratio) for electron-donating substituents on the aromatic aldehydes.
3.6. Nucleophilic addition of acetaldehyde to diyne system
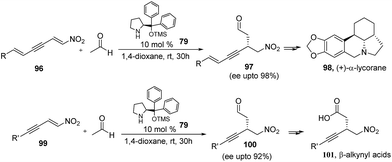
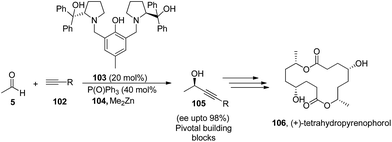
Importance of acetaldehyde can also be visualized from its application in the synthesis of biologically active compounds like (+)-α-lycorane and chiral β-alkynyl acids. Xue-Ling and co workers53 used acetaldehyde in the organocatalytic reaction of nitrodienynes (96) and nitroenynes (99). The conjugate addition reaction of acetaldehyde with 96 and 99 in the presence of diarylprolinolsilyl ethers (79), affords the desired product 97 and 100 respectively in good yields as well as in high enantioselectivities. Owing to various functionalities present in a single molecule, authors have successfully used their reaction product in enantioselective total synthesis of (+)-α-lycorane (98), which belongs to a alkaloid family, widely known for their different biological activities such as antiviral, antineoplastic, inhibitor of cell growth and cell division in higher plants.54 Finally they have shown that the product can also be converted to chiral β-alkynyl acids (101), which represent an important class of pharmaceutical compounds having wide range of biological activities like PDE IV inhibitors, TNF inhibitors, GPR40 receptor agonists, and GRP receptor antagonists55(Scheme 21).Trost and Quintard56 have used acetaldehyde for organocatalytic asymmetric alkynylation to access propargylic alcohols. The between (1) and (102) reaction in the presence of catalyst (S,S) ProPhenol/POPh3 (103) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −20 °C resulted the product (105) in 78% yield and 86% enantioselectivity. Since the reaction was suffering with self aldol side reaction, therefore to overcome the problem, rate of addition of acetaldehyde in reaction mixture was studied, where slow addition over 30 minutes was found to be the condition of choice which resulted in high product formation and reduces the chances of self aldol reaction (Scheme 22).
1) at −20 °C resulted the product (105) in 78% yield and 86% enantioselectivity. Since the reaction was suffering with self aldol side reaction, therefore to overcome the problem, rate of addition of acetaldehyde in reaction mixture was studied, where slow addition over 30 minutes was found to be the condition of choice which resulted in high product formation and reduces the chances of self aldol reaction (Scheme 22).
4. Enolactetate as indirect source of acetaldehyde in asymmetric transformations
To overcome the problems encountered in the direct use of acetaldehyde, a lot of research is ongoing where acetaldehyde has been generated in situ from stable and easy to handle chemicals. Paraldehyde,57 vinyl acetate,58 and vinyl ethers59 are being mostly used as the indirect sources of acetaldehyde in asymmetric organic transformations.4.1. Vinyl acetate in cross-aldol reaction
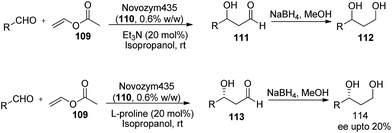
Since the direct use of acetaldehyde generally suffers with various side uncontrolled reaction. Also, some of the authors suggested that slow addition of acetaldehyde can overcome the problem of high reactivity of acetaldehyde, this can also be achieved by in situ generation of acetaldehyde in reaction mixture. Since in the past vinyl acetate is well known for in situ generation of acetaldehyde during the transesterification of hydroxyl compounds60 but the first report of vinyl acetate in aldol condensation appears when Gupta and coworkers61 were trying for asymmetric transesterification of tricyclic diketone in presence of Novozym 435 lipase. Instead of getting the expected transacetylated product they got the condensation product of tricyclic ketone and acetaldehyde. Reaction involves first enzyme-catalysed nucleophilic addition of (1) with acetaldehyde resulting in the formation of mono-adduct which further undergoes second condensation reaction (1) to afford the bis-adduct in 97% yield, although in racemic fashion.In 2011, our group reported the first attempt on the use of vinyl acetate (109) in asymmetric cross-aldol reaction where lipase was used in tandem with organocatalyst.62 The reaction apparently involves lipase (110) catalyzed in situ generation of active form of acetaldehyde from vinyl acetate followed by organocatalysed aldol reaction with aromatic aldehydes for the preparation of β-hydroxy aldehydes. In the initial studies reaction was studied with triethylamine in tandem with lipase where the product (111) obtained in good yields but is racemic in nature. After the initial success, reaction was tested for tolerance of the lipase with organocatalyst towards variously substituted aromatic aldehydes. The reaction of in the presence of L-proline and lipase proceeded well and afforded the corresponding product (113) in moderate yields (42–65%) but in quite low enantioselectivity ranging between 15–20%.
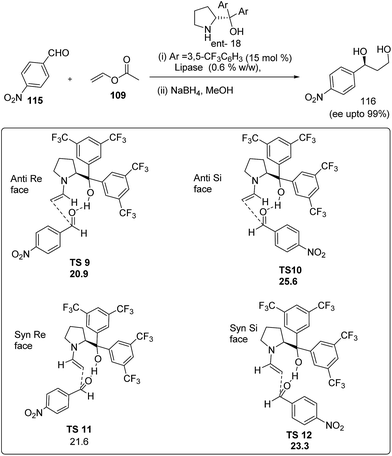
To further explore the use of vinyl acetate as an alternative source of acetaldehyde our group have recently employed vinyl acetate in for highly enantioselective cross-aldol reaction63 (Scheme 24). The reaction apparently involves in situ generation of acetaldehyde from vinyl acetate through lipase Novozym 435 followed by the cross aldol reaction in the presence of bis-trifluoro substituted diphenylprolinol (18). Under the optimized conditions, reaction between acetaldehyde and various aromatic aldehydes afford the cross-aldol product in good yields (60–94%) and high enantioselectivities (up to 99%). Reaction mechanism was proposed by performing density functional energy (DFT) calculations. Four possible transition sates were studied i.e. anti–Re face (TS9), anti–Si face (TS10), syn–Re face (TS11) and syn–Si (TS-12) face. All the transition state involves hydrogen bonding between the acidic OH group of the trifluoromethyl-substituted catalyst (18) and the carbonyl group of acetaldehyde, which activates the substrate for reaction as well as controlling the direction of approach. DFT calculation predict the most stable transition state as (TS9) where the aryl rings of the aldehyde and catalyst are sufficiently far apart resulted in the formation of stable adduct having minimum energy (20.9 kcal mol−1). Also the synthetic importance of cross-aldol product is well addressed in the asymmetric synthesis of α,β-unsaturated δ-lactones, a well known structural motif present in a wide variety of bioactive natural scaffolds.64
A simple, mild, one-pot tandem method catalyzed by trypsin was developed65 for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (119) by the Biginelli reaction of urea (117), β-dicarbonyl compounds (118), and in situ-formed acetaldehyde from vinyl acetate (109). Trypsin was found to display dual promiscuous functions to catalyze transesterification and the Biginelli reaction in sequence (Scheme 25).
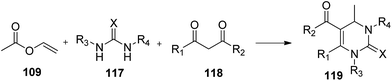 | ||
| Scheme 25 Trypsin-catalyzed tandem reaction: one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones by in situ formed acetaldehyde. | ||
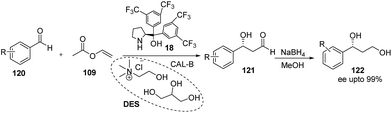
Recently Maria and coworkers66 have reported the asymmetric cross aldol reaction of acetaldehyde in deep eutectic solvent (DES) i.e. choline chloride and glycerol, by using lipase Cal-B and proline derived organocatalysts. Screening of organocatalysts showed that trifluoromethyl substituted proline catalyst (18) is the most suitable organocatalyst for this transformation and afford the product (121) in 70% yield and 95% enantiomeric excess. Author have successfully employed the DES solvent with lipase, where both can be reused up to 6 cycles without any loss of activity, while fresh organocatalyst is required every time for good results.
4.2. Paraldehyde in asymmetric Michael reaction
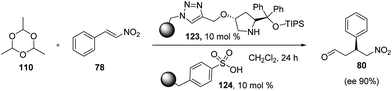
After the successful employment of vinyl acetate as an indirect source of acetaldehyde, Pericas et al.67 recently showed the use of cyclic trimer paraldehyde (110), as a precursor for acetaldehyde. Since its more stable liquid as compared to acetaldehyde, thereby reduces the challenges associated with handling of acetaldehyde. The reaction involves the acid-catalyzed depolymerisation of paraldehyde (110), which in situ generates acetaldehyde and further in the presence of amino-catalyst undergoes Michael reaction. Since both catalysts were incompatible to each other so authors tried to exploit the site isolation principle, where polystyrene supported catalysts (123) and (124) were used in the reaction of paraldehyde and trans-β-nitrostyrene. As expected both the catalysts corporate with each other and affords the product (80) in 42% yield and 90% ee. Reaction also shows the improved results when 123 were confined to teabag in CH2Cl2 solvent (Scheme 27).5. Conclusions
In summary, this review summarises both the recent developments in the field of organocatalysed use of acetaldehyde as a substrate in various organic transformations and the application of acetaldehyde derived products in the synthesis of bioactive molecules. Inspite the parallel development of acetaldehyde as an efficient substrate, the discovery of highly effective organocatalysts for nucleophilic addition of acetaldehyde to the various electrophilic centres was undoubtedly a momentous point for recent development of the field. We hope that this short review will further stimulate collective thinking on design, synthesis of organocatalysts and new approaches for stereocenter forming reactions involving unexplored substrates, to increase the molecular diversity in organic transformations.Acknowledgements
We thank DST, New Delhi for financial assistance. Also M.K. thanks CSIR, New Delhi for the award of Research Fellowship. IIIM Communication no. 1802.Notes and references
- (a) T. Hayashi and M. Kumada, Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, Inc., 1985, vol. 5, p. 147 Search PubMed; (b) R. Mahrwald, Enantioselective Organocatalyzed Reactions II: Asymmetric C-C Bond Formation Processes, Springer, New York, 2011 Search PubMed.
- (a) U. Scheffler and R. Mahrwald, Chem.–Eur. J., 2013, 19, 14346 CrossRef CAS PubMed; (b) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626 CrossRef CAS PubMed.
- (a) A. N. Collins, G. N. Sheldrake and J. Crosby, Chirality in Industry II, Wiley, New York, 1997 Search PubMed; (b) G. Beck, Synlett, 2002, 6, 837 CrossRef.
- (a) R. Mahrwald, Modern Aldol Reactions, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2004, vol. 1 and 2 Search PubMed; (b) C. H. Heathcock, in Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, Orlando, FL, 1984, vol. 3, p. 111 Search PubMed; (c) C. H. Heathcock, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 2, p. 133 Search PubMed; (d) D. Gryko and D. Walaszek, Stereoselective Organocatalysis: Bond Formation Methodologies and Activation Modes, ed. R. R. Torres, John Wiley & Sons, Inc., Hoboken, New Jersey, ch. 3, 2013 Search PubMed; (e) T. D. Machajewski and C.-H. Wong, Angew. Chem., Int. Ed., 2000, 39, 1352 CrossRef CAS; (f) C. Palomo, M. Oiarbide and J. M. Garcia, Chem. Soc. Rev., 2004, 33, 65 RSC.
- (a) K. C. Nicolaou, S. A. Snyder, T. Montagnon and G. Vassilikogiannakis, Angew. Chem., Int. Ed., 2002, 41, 1668 CrossRef CAS; (b) J. A. Norton, Chem. Rev., 1942, 42, 319 CrossRef; (c) R. Huisgen, R. Grashey and J. Sauer, in The Chemistry of the Alkenes, ed. S. Patai, Wiley, London, 1964, p. 739 Search PubMed; (d) D. A. Evans and J. S. Johnson, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, New York, 1999, vol. 3, p. 1177 Search PubMed; (e) W. Oppolzer, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon Press, New York, 1991, vol. 5 Search PubMed; (f) H. B. Kagan and O. Riant, Chem. Rev., 1992, 92, 1007 CrossRef CAS.
- (a) S. C. Jha and N. N. Joshi, ARKIVOC, 2002, 7, 167 CrossRef; (b) M. E. Jung, Stabilized Nucleophiles with Electron Deficient Alkenes and Alkynes, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Elmsford, NY, 1991, vol. 4, ch. 1.1 Search PubMed; (c) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon Press, Elmsford, NY, 1992 Search PubMed; (d) Y. Zhang and W. Wang, Catal. Sci. Technol., 2012, 2, 42 RSC.
- (a) T. Graening and H. G. Schmalz, Angew. Chem., Int. Ed., 2003, 42, 2580 CrossRef CAS PubMed; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (c) B. M. Trost and T. J. Fullerton, J. Am. Chem. Soc., 1973, 95, 292 CrossRef CAS; (d) B. M. Trost and D. L. V. Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed; (e) B. M. Trost, Org. Process Res. Dev., 2012, 16, 185 CrossRef CAS PubMed.
- (a) M. Ueno and S. Kobayashi, Enantioselective Synthesis of β-Amino Acids, ed. E. Juaristi and V. A. Soloshonok, Wiley-VCH, New York, 2nd edn, 2005, p. 139 Search PubMed; (b) J. M. Verkade, L. J. Vhemert, P. J. Quaedflieg and F. P. Ruties, Chem. Soc. Rev., 2008, 37, 29 RSC; (c) A. Cordova, Acc. Chem. Res., 2004, 37, 102 CrossRef CAS PubMed.
- (a) P. Langer, Angew. Chem., Int. Ed., 2000, 39, 3049 CrossRef CAS; (b) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS PubMed; (c) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (d) F. L. Hu and M. Shi, Org. Chem. Front., 2014, 1, 587 RSC.
- (a) S. G. Davies, Organotransition Metal Chemistry Applications to organic Synthesis, Pergamon, Oxford, 1982 Search PubMed; (b) E. Meggers, Chem.–Eur. J., 2010, 752 CrossRef CAS PubMed; (c) B. G. Davis and V. Boyer, Nat. Prod. Rep., 2001, 18, 618 RSC; (d) S. I. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC.
- (a) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed; (b) M. J. Gaunt, C. C. Johansson, A. McNally and N. T. Vo, Drug Discovery Today, 2007, 12, 1 CrossRef PubMed; (c) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed; (d) J. Alemán and S. Cabrera, Chem. Soc. Rev., 2013, 42, 774 RSC; (e) M. C. Holland and R. Gilmour, Angew. Chem., Int. Ed., 2015, 54, 3862 CrossRef CAS PubMed; (f) F. G. Finelli, L. S. M. Miranda and R. O. M. A. de Souza, Chem. Commun., 2015, 51, 3708 RSC.
- (a) D. W. C. MacMillan, Nature, 2008, 455, 304 CrossRef CAS PubMed; (b) S. V. Pansare and E. K. Paul, Chem.–Eur. J., 2011, 17, 8770 CrossRef CAS PubMed; (c) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed; (d) U. Scheffler and R. Mahrwald, Chem.–Eur. J., 2013, 19, 14346 CrossRef CAS PubMed; (e) A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638 CrossRef CAS PubMed.
- Z. G. Hajos and D. R. Parrish, in Ger. Pat. DE 21026, 29 July 1971transJ. Org. Chem., 1974, 39, 1615 Search PubMed.
- U. Eder, G. Sauer and R. Wiechert, in Ger. Pat. DE 2014757, 7 October 1971transAngew. Chem., Int. Ed. Engl., 1971, 10, 496 Search PubMed.
- B. List, R. A. Lerner and C. F. Barbas, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS.
- (a) B. Alcaide and P. Almendros, Angew. Chem., Int. Ed., 2008, 47, 4632 CrossRef CAS PubMed; (b) S. M. Kim, Synlett, 2014, 25, 153 CrossRef CAS PubMed.
- (a) L. G. Wade, Organic Chemistry, Prentice Hall, Upper Saddle River, New Jersey, 6th edn, 2005, p. 1056 Search PubMed; (b) M. B. Smith and J. March, Advanced Organic Chemistry, Wiley Interscience, New York, 5th edn, 2001, p. 1218 Search PubMed.
- (a) B. M. Kim, S. F.Williams and S. Masamune, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 2, p. 239 Search PubMed; (b) I. Paterson, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 2, p. 301 Search PubMed; (c) C. Gennari, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 2, p. 629 Search PubMed; (d) M. Braun, in Stereoselective Synthesis(Houben-Weyl, Methods of Organic Chemistry), ed. G. Helmchen, R. W. Hoffmann, J. Mulzer and E. Schaumann, George Thieme Verlag, Stuttgart, 1995, vol. E21b, p. 1603 Search PubMed; (e) A. S. Franklin and I. Paterson, Contemp. Org. Synth., 1994, 1, 317 RSC.
- A. Cordova, W. Notz and C. F. Barbas, J. Org. Chem., 2002, 67, 301 CrossRef CAS PubMed.
- (a) G. E. Keck, A. Palani and S. F. McHardy, J. Org. Chem., 1994, 59, 3113 CrossRef CAS; (b) L. R. Hillis and R. C. Ronald, J. Org. Chem., 1985, 50, 470 CrossRef CAS; (c) C. J. Hayes and C. H. Heathcock, J. Org. Chem., 1997, 62, 2678 CrossRef CAS.
- S. E. Denmark and T. Bui, J. Org. Chem., 2005, 70, 10190 CrossRef CAS PubMed.
- Y. Hayashi, S. Samanta, T. Itoh and H. Ishikawa, Org. Lett., 2008, 10, 5581 CrossRef CAS PubMed.
- (a) C. Mannich and W. Krosche, Arch. Pharm., 1912, 250, 647 CrossRef CAS PubMed; (b) S. Kobayashi and M. Ueno, in Comprehensive Asymmetric Catalysis, Supplement I, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 2003, ch. 29.5 Search PubMed; (c) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (d) M. M. B. Marques, Angew. Chem., Int. Ed., 2006, 45, 348 CrossRef CAS PubMed; (e) M. Shibasaki and S. Matsunaga, J. Organomet. Chem., 2006, 691, 2089 CrossRef CAS PubMed; (f) A. Cordova, Acc. Chem. Res., 2004, 37, 102 CrossRef CAS PubMed.
- J. W. Yang, C. Chandler, M. Stadler, D. Kampen and B. List, Nature, 2008, 452, 453 CrossRef CAS PubMed.
- (a) F. A. Davis and J. M. Szewczyk, Tetrahedron Lett., 1998, 39, 5951 CrossRef CAS; (b) G. B. Fodor and B. Colasanti, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1985, pp. 1–90 Search PubMed.
- (a) P. Dorr, M. Westby, S. Dobbs, P. Griffin, B. Irvine, M. Macartney, J. Mori, G. Rickett, C. S. Burchnell, C. Napier, R. Webster, D. Armour, D. Price, B. Stammen, A. Wood and M. Perros, Antimicrob. Agents Chemother., 2005, 49, 4721 CrossRef CAS PubMed; (b) D. A. Price, S. Gayton, M. D. Selby, J. Ahman, S. H. Lewandowski, B. L. Stammen and A. Warren, Tetrahedron Lett., 2005, 46, 5005 CrossRef CAS PubMed.
- (a) L. A. Sorbera, J. Castaner and R. M. Castaner, Drugs Future, 2004, 29, 1201 CrossRef CAS; (b) S. A. Siddiqui and K. V. Srinivasan, Tetrahedron: Asymmetry, 2007, 18, 2099 CrossRef CAS PubMed.
- S. G. Davies, A. C. Garner, P. M. Roberts, A. D. Smith, M. J. Sweeta and J. E. Thomson, Org. Biomol. Chem., 2006, 4, 2753 CAS.
- (a) P. V. Ramachandran and T. E. Burghardt, Chem.–Eur. J., 2005, 11, 4387 CrossRef CAS PubMed; (b) H. S. Lee, J. S. Park, B. M. Kim and S. H. Gellman, J. Am. Chem. Soc., 2007, 129, 6050 CrossRef PubMed.
- Y. Hayashi, T. Okano, T. Itoh, T. Urushima, H. Ishikawa and T. Uchimaru, Angew. Chem., Int. Ed., 2008, 47, 9053 CrossRef CAS PubMed.
- Q. Chang, J. Zhou and L.-H. Gan, J. Phys. Org. Chem., 2012, 25, 667 CrossRef CAS PubMed.
- C. Chandler, P. Galzerano, A. Michrowska and B. List, Angew. Chem., Int. Ed., 2009, 48, 1978 CrossRef CAS PubMed.
- T. Kano, Y. Yamaguchi and K. Maruoka, Angew. Chem., Int. Ed., 2009, 48, 1838 CrossRef CAS PubMed.
- Y. Hayashi, T. Itoh, S. Aratake and H. Ishikawa, Angew. Chem., Int. Ed., 2008, 47, 2082 CrossRef CAS PubMed.
- N. Hara, S. Nakamura, N. Shibata and T. Toru, Chem.–Eur. J., 2009, 15, 6790 CrossRef CAS PubMed.
- T. Itoh, H. Ishikawa and Y. Hayashi, Org. Lett., 2009, 11, 3854 CrossRef CAS PubMed.
- Q. Guo and J. C.-G. Zhao, Tetrahedron, 2012, 53, 1768 CrossRef CAS PubMed.
- W.-B. Chen, X.-L. Du, L.-F. Cun, X.-M. Zhang and W.-C. Yuan, Tetrahedron, 2010, 66, 1441 CrossRef CAS PubMed.
- (a) S. Pizzarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef CAS PubMed; (b) Z. Jiang, Z. Liang, X. Wu and Y. Lu, Chem. Commun., 2006, 2801 RSC; (c) T. Kanemitsu, A. Umehara, M. Miyazaki, K. Nagata and T. Itoh, Eur. J. Org. Chem., 2011, 2011, 993 CrossRef PubMed.
- S. Hu, L. Zhang, J. Li, S. Luo and J.-P. Cheng, Eur. J. Org. Chem., 2011, 2011, 3347 CrossRef CAS PubMed.
- Y. Qiao, Q. Chen, S. Lin, B. Ni and A. D. Headley, J. Org. Chem., 2013, 78, 2693 CrossRef CAS PubMed.
- S. Perera, V. K. Naganaboina, L. Wang, B. Zhang, Q. Guo, L. Rout and C.-G. Zhao, Adv. Synth. Catal., 2011, 353, 1729 CrossRef CAS PubMed.
- (a) T. Komnenos, Liebigs Ann. Chem., 1883, 218, 145 CrossRef PubMed; (b) A. Michael, J. Prakt. Chem., 1887, 36, 349 Search PubMed; (c) B. E. Rossiter and N. M. Swingle, Chem. Rev., 1992, 92, 771 CrossRef CAS; (d) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon Press, Oxford, 1992 Search PubMed.
- P. García-García, A. Ladépêche, R. Halder and B. List, Angew. Chem., Int. Ed., 2008, 47, 4722 CrossRef PubMed.
- Y. Hayashi, T. Itoh, S. Aratake and H. Ishikawa, Angew. Chem., Int. Ed., 2008, 47, 2082 CrossRef CAS PubMed.
- (a) R. H. Dworkin and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2005, 4, 455 CrossRef CAS PubMed; (b) P. J. Siddall, M. J. Cousins, A. Otte, T. Griesing, R. Chambers and T. K. Murphy, Neurology, 2006, 67, 1792 CrossRef CAS PubMed.
- A. Muzyk, S. Rivelli and J. Gagliardi, CNS Drugs, 2012, 26, 69 CrossRef CAS PubMed.
- (a) J. Zhu, E. Mix and B. Winblad, CNS Drug Rev., 2001, 7, 387 CrossRef CAS PubMed; (b) A. L. L. Garcia, M. J. S. Carpes, A. C. B. M. de Oca, M. A. G. dos Santos, C. S. C. Santan and C. R. D. Correia, J. Org. Chem., 2005, 70, 1050 CrossRef CAS PubMed.
- (a) J. L. Watts, S. A. Salmon, M. S. Sanchez and R. J. Yancey Jr, Antimicrob. Agents Chemother., 1997, 41, 1190 CAS; (b) J. M. Domagala, S. E. Hagen, T. Joannides, J. S. Kiely, E. Laborde, M. C. Schroeder, J. A. Sesnie, M. A. Shapiro, M. J. Suto and S. Vanderroest, J. Med. Chem., 1993, 36, 871 CrossRef CAS; (c) J. Katarzynska, A. Mazur, M. Bilska, E. Adamek, M. Zimecki, S. Jankowski and J. Zabrocki, J. Pept. Sci., 2008, 14, 1283 CrossRef CAS PubMed; (d) Y. J. Kim, D. A. Kaiser, T. D. Pollard and Y. Ichikawa, Bioorg. Med. Chem. Lett., 2000, 10, 2417 CrossRef CAS.
- (a) E. M. Geertsema, Y. Miao, P. G. Tepper, P. de Haan, E. Zandvoort and G. J. Poelarends, Chem.–Eur. J., 2013, 19, 14407 CrossRef CAS PubMed; (b) E. Zandvoort, E. M. Geertsema, B.-J. Baas, W. J. Quax and G. J. Poelarends, Angew. Chem., Int. Ed., 2012, 51, 1240 CrossRef CAS PubMed.
- S. M. Kim, M. Y. Jin, M. J. Kim, Y. Cui, Y. S. Kim, L. Zhang, C. E. Song, D. H. Ryu and J. W. Yang, Org. Biomol. Chem., 2011, 9, 2069 CAS.
- M. Y. Jin, S. M. Kim, H. Han, D. H. Ryu and J. W. Yang, Org. Lett., 2011, 13, 880 CrossRef CAS PubMed.
- X.-L. Meng, T. Liu, Z.-W. Sun, J.-C. Wang, F.-Z. Peng and Z.-H. Shao, Org. Lett., 2014, 16, 3044 CrossRef CAS PubMed.
- O. Hoshino, in The Alkaloids, ed. G. A. Cordell, Academic Press, New York, 1998 Search PubMed.
- (a) T. Shimada, H. Ueno, K. Tsutsumi, K. Aoyagi, T. Manabe, S. Y. Sasaki and S. Katoh, Int Appl PCT, WO 2009054479, 2009; (b) J. Houze, J. Liu, Z. Ma, J. C. Medina, M. J. Schmitt, R. Sharma, Y. Sun, Y. Wang and L. Zhu, US Pat. 7,465,804, 2008; (c) S. P. Brown, P. Dransfield, Z. Fu, J. Houze, X. Y. Jiao, T. J. Kohn, V. Pattaropong, M. Vimolratana and M. J. Schmitt, Int Appl PCT, WO 2008130514, 2008; (d) S. B. Bharate, K. V. Nemmani and R. A. Vishwakarma, Expert Opin. Ther. Pat., 2009, 19, 237 CrossRef CAS PubMed.
- A. Quintard and B. M. Trost, Angew. Chem., 2012, 124, 6808 CrossRef PubMed.
- T. V. Vasudevan and M. Sharma, Ind. Eng. Chem. Process Des. Dev., 1983, 22, 161 CAS.
- D. Mukherjee, B. A. Shah, P. Gupta and S. C. Taneja, J. Org. Chem., 2007, 72, 8965 CrossRef CAS PubMed.
- M. B. Boxer and H. Yamamoto, J. Am. Chem. Soc., 2006, 128, 48 CrossRef CAS PubMed.
- (a) U. Alder, D. Breitgoff, P. Klein, K. Laumen and P. Schneirder, Tetrahedron Lett., 1989, 30, 1793 CrossRef; (b) A. M. Brzozowski, U. Derewenda, Z. S. Derewenda, G. G. Dodson, D. M. Lawson, J. P. Turkenburg, F. Bjorkling, B. H. Jensen, S. A. Patkar and L. Thim, Nature, 1991, 351, 91 CrossRef PubMed.
- A. B. Majumder, N. G. Ramesh and M. N. Gupta, Tetrahedron Lett., 2009, 50, 5190 CrossRef CAS PubMed.
- M. Kumar, B. A. Shah and S. C. Taneja, Adv. Synth. Catal., 2011, 353, 1207 CrossRef CAS PubMed.
- M. Kumar, A. Kumar, M. Rizvi, M. Mane, K. Vanka, S. C. Taneja and B. A. Shah, Eur. J. Org. Chem., 2014, 24, 5247 CrossRef PubMed.
- (a) M. A. Koch, A. Schuffenhauer, M. Scheck, S. Wetzel, M. Ca-saulta, A. Odermatt, P. Ertl and H. Waldmann, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17272 CrossRef CAS PubMed; (b) V. Boucard, G. Broustal and J. M. Campagne, Eur. J. Org. Chem., 2007, 225 CrossRef CAS PubMed; (c) P. Barbier and F. Schneider, J. Org. Chem., 1988, 53, 1218 CrossRef CAS; (d) Q. Zhu, L. Qiao, Y. Wu and Y.-L. Wu, J. Org. Chem., 2001, 66, 2692 CrossRef CAS PubMed.
- Z. B. Xie, N. Wang, W. X. Wu, Z. G. Le and X. Q. Yu, J. Biotechnol., 2014, 170, 1 CrossRef CAS PubMed.
- C. R. Muller, I. Meiners and P. D. Maria, RSC Adv., 2014, 4, 46097 RSC.
- C. R. E. X. Fan, S. Sayalero and M. A. Pericas, Chem.–Eur. J., 2013, 19, 10814 CrossRef PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |