Fluffy-ball-shaped carbon nanotube–TiO2 nanorod nanocomposites for photocatalytic degradation of methylene blue†
Zhisong Lu*abc,
Xiutao Xiangabc,
Long Zouabc and
Jiale Xieabc
aChongqing Key Laboratory for Advanced Materials & Technologies of Clean Energies, Southwest University, 1 Tiansheng Road, Chongqing 400715, P. R. China. E-mail: zslu@swu.edu.cn; Fax: +86 23 68254969; Tel: +86 23 68254732
bInstitute for Clean Energy & Advanced Materials, Southwest University, 1 Tiansheng Road, Chongqing 400715, P. R. China
cFaculty of Materials & Energy, Southwest University, 1 Tiansheng Road, Chongqing 400715, P. R. China
First published on 5th May 2015
Abstract
One dimensional TiO2 nanomaterials have attracted tremendous attention due to their excellent photocatalytic properties. However, the synthesis of spherical-shaped carbon nanotubes (CNTs)–TiO2 nanorod composites for photocatalytic degradation of water pollutants has not been reported. In the present study we fabricated fluffy-ball-shaped multiwalled CNT–TiO2 nanorod composites via a facile hydrothermal approach. By using scanning electron microscopy (SEM) and transmission electron microscopy (TEM), it is found that morphologies of the nanocomposites could be controlled by changing the reaction duration, CNT amount and Ti source concentration. TEM images and X-ray powder diffraction (XRD) results show the excellent crystalline structure and the rutile phase of the TiO2 nanorods in the nanocomposites. Based on the results, a possible mechanism for the growth of the nanocomposites was proposed. Great potentials of the composited microspheres in water treatment were demonstrated through the photocatalytic degradation of methylene blue, in which a degradation efficiency as high as 93% could be reached. This study provides not only a new approach to developing CNT–TiO2 nanorod composites, but also a very promising photocatalyst for potential applications in waste water treatment.
Introduction
In the past decade titanium dioxide (TiO2) has been widely used in various applications such as self-cleaning surface coating,1,2 solar energy harvesting,3 gas sensor4 and water treatment5 due to its excellent semiconducting properties. Among them, the use of TiO2 for waste water treatment, in particular water pollutant degradation,6–8 attracts tremendous interests of researchers because of its low cost, good stability and great biocompatibility.9–12 During a typical photo-degradation process, light irradiation could trigger the charge separation on TiO2 surface, leading to the oxidation of adsorbents via a photocatalytic reaction. Therefore, adsorption of contaminants is a very essential procedure that allows the direct contact of contaminants with photo-induced surface oxidants.In order to achieve a high adsorption capacity, the structure of TiO2 has been tailored at nanoscale to provide large specific surface area. TiO2 nanoparticles with controllable size were synthesized to prepare ultra-transparent particulate TiO2 films with enhanced photocatalytic efficiency.13 An effective photo-oxidation was demonstrated with a suspension of 30–160 nm sized TiO2 microspheres.14 Besides the particulate shape, TiO2 nanomaterials with mesocrystal nanosheet morphology and interconnected structures were also fabricated for the photocatalytic decomposition of organic dyes.15,16 In recent years the interest in one-dimensional (1-D) TiO2 nanomaterials has been growing in both academic and industrial fields. In addition to the large surface area, 1-D TiO2 can provide confined transport of electrons, great mechanical strength and excellent flexibility, which render them the capability to be manipulated into various shapes. So far, TiO2 nanomaterials with fiber,17,18 tube19,20 and rod21,22 structures have already been developed to obtain better photocatalytic properties.
Since photo-induced charge separation on TiO2 surface is the phenomenon at the base of the photocatalytic water decontamination, the challenge is how to retard the recombination of electron–hole pairs for high photocatalytic efficiency. To conquer it, great efforts have been dedicated to hybrid TiO2 with carbon materials, which are utilized as supports for TiO2 owing to their excellent thermal,23 optical,24 mechanical25 and electrical26 characteristics. More importantly, carbon material could act as an electron sink to accept the injection of electrons from light-excited TiO2, while hindering the charge recombination and maintaining the holes to promote the contaminants oxidation process.27 Carbon nanotube (CNT) is a nanoscale 1-D carbon material with large specific surface area, high quality active sites and excellent flexibility.28 It has been composed with TiO2 to form CNT–TiO2 nanocomposites for the enhancement of photocatalytic activities. CNT–TiO2 nanocomposites with morphologies including mesoporous TiO2 mesocrystal-coated CNT,29 CNT-embedded TiO2 microspheres30 and TiO2 nanocrystal-covered CNT thin film31,32 have been fabricated with different approaches. However, the TiO2 shows particulate shapes in those nanocomposites.
Due to the advantages of 1-D structured TiO2 over particulate shaped ones, the combination of CNT with 1-D TiO2 nanomaterials has been fueled up. Direct mixing of CNT with 1-D structured TiO2 (ref. 33) and simple coating of CNT on TiO2 nanotubes34 are two widely used methods for the preparation of nanocomposites. By using them the ratio of components in the composite can be easily controlled, but the insufficient contact between CNT and TiO2 may limit the electron transfer performance. Very recently, single-crystalline 1-D TiO2–CNT nanocomposites with well connected network structures were in situ synthesized and applied in dye-sensitized solar cells.35 As to our knowledge, in situ synthesis of CNT–1-D TiO2 nanocomposites with other morphology, in particular spherical shape, for the photocatalytic degradation has not been realized.
In the present study fluffy-ball-shaped CNT–TiO2 nanorod composites were prepared with a hydrothermal approach. After calcination at 800 °C for 2 hours, the products were characterized using scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), transmission electron microscopy (TEM), X-ray powder diffraction (XRD) and thermogravimetric analysis (TGA). Based on the characterization data a mechanism was proposed to explain the formation process. The photocatalytic degradation performance of the nanocomposites was also demonstrated with methylene blue (MB) as a model pollutant.
Experimental section
Reagents
Methylene blue was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tetrabutyl titanate (≧99.0%) and titanium tetrachloride (99.9% metals basis) were bought from Aladdin (Shanghai, China). Multi-walled CNTs (MWCNTs) were purchased from Shenzhen Nanotech Port Co. Ltd. (L-MWNT-1020, 10–20 nm in diameter, 5–15 μm in length, purity > 97%, Shenzhen, China). All other chemicals are of analytical grade and used as received without further purification in this work. All solutions were prepared with deionized (DI) water (≧18 MΩ cm) generated from a Millipore Q water purification system.Preparation of CNT–TiO2 nanorod nanocomposites
A typical preparation method is as follows: 7.0 mg MWCNTs were dispersed via sonication in 10 mL toluene for 30 min. After that, the suspension was transferred into a 50 mL Teflon-lined stainless steel autoclave. 1 mL of tetrabutyl titanate, 1 mL of 37 wt% HCl and 500 μL of titanium tetrachloride were added into the autoclave in sequence. The reaction was conducted at 180 °C for 140 min. After cooling down to room temperature the product was separated and washed with ethanol for three times by centrifugation (6000 rpm, 5 min). Finally, the CNT–TiO2 nanocomposites were calcined at 800 °C for 2 hours in nitrogen atmosphere. Amounts of MWCNT and titanium tetrachloride, as well as the hydrothermal reaction duration, were changed respectively to explore the synthesis mechanism.Material characterizations
SEM samples were prepared by dropping 10 μL of composite suspension on the smooth side of an aluminium film, drying out under vacuum for 12 hours. SEM images of MWCNT–TiO2 nanocomposites were obtained using a JSM-6510LV electron microscope (JEOL, Tokyo, Japan) and a JSM-6700F field emission electron microscope (JEOL, Tokyo, Japan). EDS (INCA X-Max 250) spectra were recorded to analyze the chemical elements of each sample during SEM tests. TEM image was captured using a JEM-2100 electron microscope (JEOL, Tokyo, Japan) operating at 200 kV with different magnifications. XRD spectra were collected using a Cu-Kα-ray with tube conditions of 40 kV and 30 mA for 2θ ranging from 15° to 80° (MAXima-X 7000, Shimadzu, Japan). TGA was conducted under an air flow at a heating rate of 20 °C min−1 (TGA-Q50, TA instruments, USA). The Brunauer–Emmett–Teller (BET) surface areas of the samples were measured by nitrogen sorption isotherms using an automatic adsorption instrument NOVA 1200e (Quantachrome, Boynton Beach, Florida).Degradation of methylene blue
100 mg CNT–TiO2 nanocomposites were suspended in a 100 mL of 10 mg L−1 MB aqueous solution. The suspension was vigorously stirred in dark for 30 min to reach sorption equilibrium, followed by an exposure to UV irradiation (wavelength: 365 nm, 14.5 μW cm−2). 4 mL of the suspension was collected at given time intervals for the following residual concentration analysis. The absorbance at 664 nm was measured to determine the MB concentration after removal of the solid by centrifugation (6000 rpm, 5 min). The photocatalytic degradation activity of fluffy-ball-shaped TiO2 nanorod microspheres without MWCNTs, which were prepared via an 800 °C treatment of the CNT–TiO2 nanocomposites in air for 2 hours, was also evaluated with the same conditions for comparison.Results and discussion
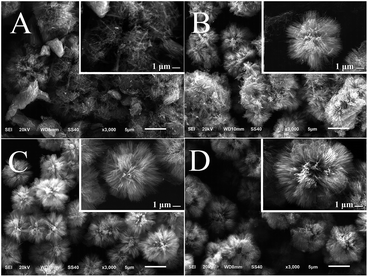
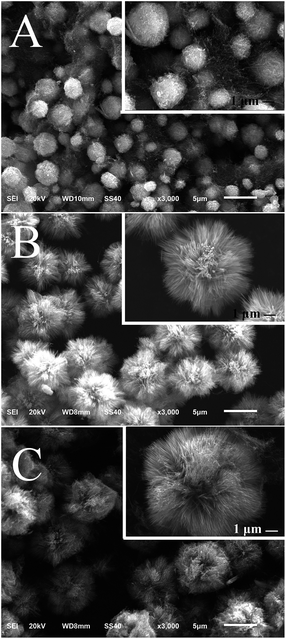
In the hydrothermal synthesis of TiO2, reaction duration is one of the important parameters that influence the morphology of final products.36 Therefore, the effect of reaction duration on the nanocomposites was examined using SEM. A network-structured matrix containing nanowires and nanoparticles is observed in Fig. 1A when the reaction duration is as short as 15 min. As the reaction duration increases to 25 min, a lot of small dots with diameter of 1–2 μm appear on the matrix (Fig. 1B). In comparison to Fig. 1B and C shows the growth and the surface density increment of the dots. Moreover, sparse nanorods can be seen on some of the dots. After a 70 min reaction fluffy-ball-shaped microspheres covered with high density of nanorods can be found and the size of the microspheres is around 5 μm (Fig. 1D). As further elongation of the reaction time, defects of the nanocomposites are made up by the nanorods. Complete fluffy-ball-shaped microspheres with the average size of ∼9 μm are finally achieved when the reaction lasts for 140 min. The SEM micrographs indicate that the morphology of the CNT–TiO2 nanocomposites can be significantly affected by the reaction duration and that a 140 min of hydrothermal reaction could produce intact fluffy-ball-shaped CNT–TiO2 rod nanocomposites with the average diameter of ∼9 μm.In order to explore the role of MWCNTs in the formation of the nanocomposites, the amount of MWCNTs was changed from 0 to 7.0 mg in the reaction system with other conditions remained constant. Amorphous structures coated with nanowires are generated in the absence of MWCNTs (Fig. 2A). The existence of MWCNTs in the system results in the formation of fluffy-ball-shaped microspheres. As the amount of CNTs increases from 2.0 mg to 7.0 mg, the nanorods become denser on the microspheres and the mean size of the nanocomposites increase from ∼7 to ∼9 μm. The results show that the addition of MWCNTs facilitates the formation of fluffy-ball-shaped nanocomposites. Both surface density of nanorods and the size of the nanocomposites are closely related to the amount of MWCNTs in the reaction system.
Titanium source is another critical parameter that needs to be considered in the synthesis of the nanocomposites. Thus, the effect of titanium source on the product structure was explored by changing the volumes of 99.9% titanium tetrachloride in the reaction system. Many smooth microspheres with the size ranging from 1 to 4 μm are generated in the reaction system containing 200 μL of titanium tetrachloride (Fig. 3A). It can be seen from the inset image of Fig. 3A that a network-structured matrix is serving as the substrate of the microspheres. The morphology is similar to that in Fig. 1B, implying that the decrease of titanium tetrachloride amount in the reaction system may slow down the reaction rate. Characteristic fluffy-ball-shaped microspheres appear when the volume of titanium tetrachloride is 350 μL (Fig. 3B). Growth of the microsphere from ∼7 to ∼9 μm is shown in Fig. 3C as the volume further rises up to 500 μL. Obviously, the volume of titanium tetrachloride in the reaction system could affect the growth of nanorods on the microspheres.
Since both CNTs and TiO2 nanorods possess 1-D morphology, it is of great importance to differentiate them and ascertain the distribution of CNTs in the nanocomposites. Fig. 4A shows the high magnified SEM image of the edge of a typical nanocomposite. A nanowire-network structure, which is quite similar to the MWCNTs networks, extends out from the microsphere. The diameter of the nanowires is much smaller than that of the nanorods in the nanocomposites. Detailed morphology of the microsphere surface is exhibited in Fig. 4B. Well-aligned straight nanorods and randomly spread nanowires tangle with each other, forming a network structure. The diameter of the nanowires is around 20 nm, which agrees well with the size of MWCNTs. From both morphology and size it is easy to identify MWCNTs in the nanocomposites. To further confirm the embedding of MWCNTs in the microspheres, the as-prepared composites were heated at 800 °C in air to remove MWCNTs from the microspheres. No nanowire can be discovered on the microspheres and some unoccupied spaces in the well-aligned nanorods appear, proving the incorporation of MWCNTs in the original CNT–TiO2 nanorod nanocomposites (Fig. 4C). Carbon element cannot be found in the EDS spectrum of TiO2 nanorod microspheres without CNTs (Fig. S1 in ESI†). However, the presence of carbon, titanium, oxygen elements in the CNT–TiO2 nanorod nanocomposites suggests the generation of TiO2 and embedding of MWCNTs (Fig. 4D). During the TEM test some of the TiO2 nanorods in the nanocomposites could be melted after a long-term irradiation with high energy electron beam, clearly showing the incorporation of MWCNTs in the microspheres (Fig. S2 in ESI†). Rutile TiO2 with 1-D morphology could be synthesized in an acidic water using TiCl4 as the Ti source. In our work we also tried to synthesize TiO2 nanocomposites using TiCl4 in acidic water along with MWCNTs. However, only some spheres can be generated (Fig. S3 in ESI†).
 | ||
| Fig. 4 SEM images of CNT–TiO2 nanorod nanocomposites (A and B) and a TiO2 nanorod microsphere after removal of MWCNT (C); (D) is a typical EDS spectrum of CNT–TiO2 nanorod nanocomposites. | ||
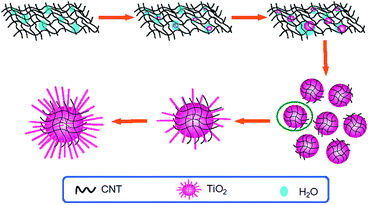
Based on above results, a mechanism for the nanocomposite growth is proposed in Scheme 1. Firstly, MWCNTs in the reaction system tangle with each other to generate a network structure. The very limited water from 37 wt% HCl tends to be entrapped in the MWCNTs network, forming a relative hydrophilic environment. Then, the hydrolysis of Ti4+ precursors occurs to produce [Ti(OH)x(OH2)6−x](4−x)+. Since the hydrolysates are only soluble in water, the water in the network can be saturated by the [Ti(OH)x(OH2)6−x](4−x)+ quickly. The condensation of the hydrolysates leads to the formation of rutile nuclei in the MWCNT matrix. Further hydrolysis of the TiO2 precursors induces the growth of the nucleus, forming bigger microsized dots to disentangle from the MWCNT network. In this process, some MWCNTs are surrounded on the surface of the dots to prevent the nucleus growing bigger. Meanwhile, since selective adsorption of Cl− on the crystal planes could induce directed growth of TiO2,37 the TiO2 nanorod is produced on the dot surface forming CNTs–TiO2 nanorod nanocomposites. As the progress of the reaction, the nanorods become longer and the size of the CNTs–TiO2 nanorod nanocomposites increases. Morphology of the nanocomposites does not change any more when the reaction reaches equilibrium.
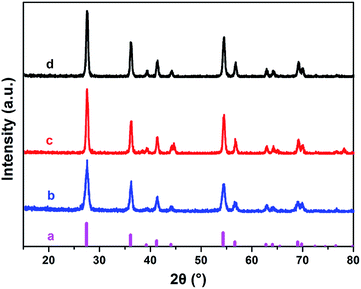
To confirm the crystallinity and phase purity of the nanocomposites, XRD measurement was carried out in this work. The rutile structure of the TiO2 in the synthesized CNT–TiO2 nanocomposites can be identified since all peaks match very well with those of the standard pattern of pure rutile TiO2 (JCPDS, no. 21-1276) (Fig. 5). It is obvious that the TiO2 possesses stronger peaks than those without calcination, indicating that calcination could enhance the crystalline degree of TiO2. Besides the peaks of rutile, no other peak is observed in all tested samples. It shows that the CNT–TiO2 nanocomposites are very pure without contaminations and can be used in the following photocatalytic degradation experiments.
TEM images were captured to provide more information about the as-prepared nanocomposites. A typical fluffy-ball shaped CNT–TiO2 nanorod nanocomposite is shown in Fig. 6A. The nanocomposite has a spherical shape and ∼8 μm diameter, which is consistent with above SEM results. Well-aligned nanorods and an interconnected CNT can be clearly seen at the edge of the microsphere (Fig. 6B). As being illustrated in Fig. 6C, the diameter of a single nanorod is around 30 nm. Fig. 6D presents a high-resolution TEM image of a nanorod. The lattice fringes with an interplanar distance of d101 = 0.245 nm corresponding to the rutile-phase of TiO2 can be clearly distinguished (Fig. 6D). The results further prove that the nature of nanorods is rutile structured TiO2 and CNT does exist in the nanocomposites. The TGA curve in Fig. S4† reveals that the weight percent of CNT in the nanocomposite is about ∼2%.
The nitrogen adsorption–desorption isotherm was measured to determine the specific surface area of TiO2 and CNT–TiO2 microspheres (Fig. 7). For the nanocomposites, a type IV isotherm is obtained, which is characteristic of mesoporous materials. The pore size distribution analysis exhibits two peaks centered at 3.6 nm and 90 nm, respectively, indicating that the nanocomposites contain both mesoporous and macroporous pores (Fig. 7A). The surface area of the CNT–TiO2 nanorod nanocomposites is calculated as 26.56 m2 g−1 based on the Brunauer–Emmett–Teller (BET) model. In comparison, a small hysteresis hoop is observed in the isotherm of TiO2 microspheres without CNTs, suggesting that the material is less porous. The first peak locating at 3.6 nm cannot be found in the pore size distribution curve of the TiO2 microspheres (Fig. 7B). The surface area of the TiO2 spheres is only 2.93 m2 g−1. Since the pore size of MWCNTs inner cavities is in the range of 3–4 nm,38 the mesopores with the pore size less than 10 nm in the CNT–TiO2 nanorod composites may mainly be contributed by inner cavities of the MWCNTs.
 | ||
| Fig. 7 Nitrogen adsorption–desorption isotherms of CNT–TiO2 nanorod composites (A) and TiO2 nanorod microspheres (B). Insets are the corresponding pore size distributions (BJH method). | ||
Photocatalytic activity of the fabricated nanocomposites was evaluated using photocatalytic degradation tests with MB as a model water contaminant (Fig. 8). Under a UV irradiation, the MB concentration in the CNT–TiO2 nanorod nanocomposites suspension reduces rapidly. After 70 min of reaction the degradation efficiency could reach to 93%, which is significantly higher than 63% degradation achieved by the CNT-embedded hollow TiO2 nanofibers.39 However, the catalytic property of the nanocomposites still cannot compete with that of commercial P25 TiO2 nanoparticles (Fig. S5 in ESI†). To investigate the role of CNTs in the photocatalytic reaction, photocatalytic degradation capability of TiO2 microspheres without CNTs was also measured. Differently from the nanocomposites, 70 min of irradiation could only lead to the degradation efficiency less than 36%. As the reaction time increase to 160 min, 64% of the MB in the suspension is removed. The data reveals that MWCNTs embedded in the CNT–TiO2 microspheres play a critical role in the photocatalytic degradation process. MWCNTs cannot catalyze the degradation of MB in dark (Fig. S6 in ESI†). As being illustrated in the SEM and TEM images, the MWCNTs randomly distribute in the well-aligned TiO2 nanorods and tangle with them to form a network structure. Because of the large electron-storage capacity of CNTs,40,41 the photon-excited electrons in TiO2 can easily flow into the connected CNTs and the holes still exist to take part in the redox reactions thus retarding the electron–hole recombination (Fig. S7 in ESI†). CNTs may facilitate the adsorption of MB on the composites via strong pi–pi interaction between CNTs and MB. The enhancement of the light absorption may also be a possible reason for the high catalytic performance of the MWCNT-laden microspheres.42,43
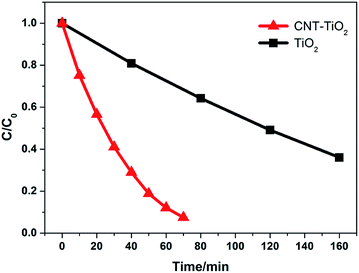 | ||
| Fig. 8 Photocatalytic degradation capabilities of TiO2 nanorod microspheres (black square) and CNT–TiO2 nanorod nanocomposites (red triangle) with MB as a model pollutant. | ||
Conclusions
In this work, fluffy-shaped CNT–TiO2 nanorod composites were fabricated via a simple one-pot hydrothermal approach. Morphologies of the nanocomposites could be controlled by changing the reaction parameters including reaction duration, CNT amount and Ti source concentration. Intact fluffy-ball-shaped CNT–TiO2 nanorod composites are obtained in a reaction system containing 7.0 mg of CNT, 10 mL of toluene, 1 mL of tetrabutyl titanate, 1 mL of 37 wt% HCl and 500 μL of titanium tetrachloride after 180 °C incubation for 140 min. MWCNTs randomly spread in the composites and tangle with well-aligned TiO2 nanorods, forming a network structure. TEM images and XRD results show the excellent crystalline structure and the rutile phase of the TiO2 nanorod in the nanocomposites. Based on the results, the growth mechanism of the CNT–TiO2 microspheres involving the nucleation of TiO2 in the CNTs network, growth of the TiO2 microspheres and the directed elongation of TiO2 nanorods was proposed. Although the weight percentage of CNTs in the nanocomposites is only ∼2%, the embedding of them could greatly increase the mesoporosity and specific surface area of the products. The photocatalytic degradation assay demonstrates that CNT–TiO2 nanorod composites could rapidly remove MB in the contaminated water and achieve 93% degradation efficiency in 70 min under a UV irradiation. This study provides not only a new approach to developing fluffy-shaped CNT–TiO2 nanorod composites, but also an efficient photocatalyst for potential applications in waste water treatment.Acknowledgements
This work is financially supported by Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies under cstc2011pt-sy90001, Start-up grant under SWU111071 from Southwest University and Chongqing Science and Technology Commission under cstc2012gjhz90002. Z. S. Lu would like to thank the supports by the Specialized Research Fund for the Doctoral Program of Higher Education (RFDP) (Grant no. 20130182120025), Chongqing Natural Science Foundation (cstc2012jjA1137) and Young Core Teacher Program of the Municipal Higher Educational Institution of Chongqing.Notes and references
- K. Nakata, M. Sakai, T. Ochiai, T. Murakami, K. Takagi and A. Fujishima, Langmuir, 2011, 27, 3275–3278 CrossRef CAS PubMed.
- T. Kamegawa, Y. Shimizu and H. Yamashita, Adv. Mater., 2012, 24, 3697–3700 CrossRef CAS PubMed.
- M. Guo, K. Y. Xie, Y. Wang, L. M. Zhou and H. T. Huang, Sci. Rep., 2014, 4, 6442 CrossRef CAS PubMed.
- C.-P. Hsu, T.-W. Zeng, M.-C. Wu, Y.-C. Tu, H.-C. Liao and W.-F. Su, RSC Adv., 2014, 4, 22926–22930 RSC.
- F. Ruggieri, A. A. D'Archivio, M. Fanelli and S. Santucci, RSC Adv., 2011, 1, 611–618 RSC.
- Y. Jiang, W. N. Wang, P. Biswas and J. D. Fortner, ACS Appl. Mater. Interfaces, 2014, 6, 11766–11774 CAS.
- P. Gao, Z. Y. Liu, M. H. Tai, D. D. Sun and W. Ng, Appl. Catal., B, 2013, 138, 17–25 CrossRef PubMed.
- X. Shao, W. C. Lu, R. Zhang and F. Pan, Sci. Rep., 2013, 3, 3018 Search PubMed.
- S. W. Leong, A. Razmjou, K. Wang, K. Hapgood, X. W. Zhang and H. T. Wang, J. Membr. Sci., 2014, 472, 167–184 CrossRef CAS PubMed.
- R. Djellabi, M. F. Ghorab, G. Cerrato, S. Morandi, S. Gatto, V. Oldani, A. Di Michele and C. L. Bianchi, J. Photochem. Photobiol., A, 2014, 295, 57–63 CrossRef CAS PubMed.
- X. Y. Liu, Q. R. Chen, L. Z. Lv, X. Y. Feng and X. F. Meng, Catal. Commun., 2015, 58, 30–33 CrossRef CAS PubMed.
- A. Y. Zhang, L. L. Long, C. Liu, W. W. Li and H. Q. Yu, Water Res., 2014, 66, 273–282 CrossRef CAS PubMed.
- S. Y. Chae, M. K. Park, S. K. Lee, T. Y. Kim, S. K. Kim and W. I. Lee, Chem. Mater., 2003, 15, 3326–3331 CrossRef CAS.
- X. Z. Li and H. Liu, Environ. Sci. Technol., 2003, 37, 3989–3994 CrossRef CAS.
- Y. Aoyama, Y. Oaki, R. Ise and H. Imai, CrystEngComm, 2012, 14, 1405–1411 RSC.
- G. Hasegawa, K. Morisato, K. Kanamori and K. Nakanishi, J. Sep. Sci., 2011, 34, 3004–3010 CrossRef CAS PubMed.
- S. H. Zhan, D. R. Chen, X. L. Jiao and C. H. Tao, J. Phys. Chem. B, 2006, 110, 11199–11204 CrossRef CAS PubMed.
- S. Chuangchote, J. Jitputti, T. Sagawa and S. Yoshikawa, ACS Appl. Mater. Interfaces, 2009, 1, 1140–1143 CAS.
- X. J. Xu, X. S. Fang, T. Y. Zhai, H. B. Zeng, B. D. Liu, X. Y. Hu, Y. Bando and D. Golberg, Small, 2011, 7, 445–449 CrossRef CAS PubMed.
- M. Sun, X. Ma, X. Chen, Y. Sun, X. Cui and Y. Lin, RSC Adv., 2014, 4, 1120–1127 RSC.
- S. Biswas, V. Sundstrom and S. De, Mater. Chem. Phys., 2014, 147, 761–771 CrossRef CAS PubMed.
- Y. W. Wang, L. Z. Zhang, K. J. Deng, X. Y. Chen and Z. G. Zou, J. Phys. Chem. C, 2007, 111, 2709–2714 CAS.
- A. A. Balandin, Nat. Mater., 2011, 10, 569–581 CrossRef CAS PubMed.
- W. A. Deheer, W. S. Bacsa, A. Chatelain, T. Gerfin, R. Humphreybaker, L. Forro and D. Ugarte, Science, 1995, 268, 845–847 CAS.
- G. D. Tarigh, F. Shemirani and N. S. Maz'hari, RSC Adv., 2015, 5, 35070–35079 RSC.
- H. C. Schniepp, J. L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS PubMed.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233–2239 CrossRef CAS PubMed.
- C. Herrero-Latorre, J. Alvarez-Mendez, J. Barciela-Garcia, S. Garcia-Martin and R. M. Pena-Crecente, Anal. Chim. Acta, 2015, 853, 77–94 CrossRef CAS PubMed.
- B. Liu and H. C. Zeng, Chem. Mater., 2008, 20, 2711–2718 CrossRef CAS.
- T. C. An, J. Y. Chen, X. Nie, G. Y. Li, H. M. Zhang, X. L. Liu and H. J. Zhao, ACS Appl. Mater. Interfaces, 2012, 4, 5988–5996 CAS.
- Y. Zhao, Y. Hu, Y. Li, H. Zhang, S. W. Zhang, L. T. Qu, G. Q. Shi and L. M. Dai, Nanotechnology, 2010, 21, 505702 CrossRef PubMed.
- H. T. Yu, X. Quan, S. Chen and H. M. Zhao, J. Phys. Chem. C, 2007, 111, 12987–12991 CAS.
- M. Daranyi, T. Csesznok, A. Kukovecz, Z. Konya, I. Kiricsi, P. M. Ajayan and R. Vajtai, Nanotechnology, 2011, 22, 195701 CrossRef PubMed.
- S. Zhang, C. Y. Ji, Z. Q. Bian, R. H. Liu, X. Y. Xia, D. Q. Yun, L. H. Zhang, C. H. Huang and A. Y. Cao, Nano Lett., 2011, 11, 3383–3387 CrossRef CAS PubMed.
- S. Sadhu and P. Poddar, J. Phys. Chem. C, 2014, 118, 19363–19373 CAS.
- J. G. Yu, G. H. Wang, B. Cheng and M. H. Zhou, Appl. Catal., B, 2007, 69, 171–180 CrossRef CAS PubMed.
- X. J. Feng, K. Shankar, O. K. Varghese, M. Paulose, T. J. Latempa and C. A. Grimes, Nano Lett., 2008, 8, 3781–3786 CrossRef CAS PubMed.
- C. Y. Lu and H. S. Chiu, Chem. Eng. Sci., 2006, 61, 1138–1145 CrossRef CAS PubMed.
- J. Y. Jung, D. Lee and Y. S. Lee, J. Alloys Compd., 2015, 622, 651–656 CrossRef CAS PubMed.
- A. Kongkanand and P. V. Kamat, ACS Nano, 2007, 1, 13–21 CrossRef CAS PubMed.
- J. G. Yu, T. T. Ma and S. W. Liu, Phys. Chem. Chem. Phys., 2011, 13, 3491–3501 RSC.
- S.-F. Leung, Q. Zhang, F. Xiu, D. Yu, J. C. Ho, D. Li and Z. Fan, J. Phys. Chem. Lett., 2014, 5, 1479–1495 CrossRef CAS.
- G. Griffini, F. Bella, F. Nisic, C. Dragonetti, D. Roberto, M. Levi, R. Bongiovanni and S. Turri, Adv. Energy Mater., 2014, 5, 1401312 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDS spectrum of the TiO2 microspheres; TEM image of MWCNTs in the CNT–TiO2 nanorod composites; SEM images of TiO2–CNT nanocomposites through using only TiCl4 in acidic water along with MWCNT; TGA curve of the CNT–TiO2 nanorod composites; photocatalytic degradation capability of P25; SEM section image of the CNT–TiO2 microspheres; UV-Vis diffuse reflectance spectra of the CNT–TiO2 microspheres and the TiO2 microspheres. See DOI: 10.1039/c5ra05641a |
| This journal is © The Royal Society of Chemistry 2015 |