Synthesis, growth, structure and characterization of potassium lithium hydrogen phthalate mixed crystals†
Abstract
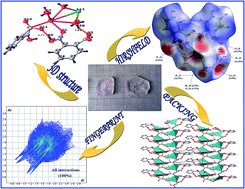
Mixed crystals of lithium-incorporated potassium hydrogen phthalate were grown by the slow evaporation solution growth technique from an aqueous solution containing equimolar quantities of potassium hydrogen phthalate (KHP) and lithium carbonate. The crystal composition, C16H16KLiO11 (PLHP), as determined by single-crystal XRD analysis reveals the coexistence of potassium and lithium in the mixed crystal, further supported by energy dispersive X-ray spectroscopy and atomic absorption spectroscopy. It belongs to the monoclinic system with the space group P21 and the cell parameters a = 9.4866(3) Å, b = 6.769(2) Å, c = 15.3967(5) Å, α = γ = 90°, β = 105.730°(3), V = 951.67(5) Å3 and. Z = 2. The relative second harmonic generation (SHG) efficiency measurements reveal that PLHP has an efficiency comparable to that of KHP. The grown crystals were further characterized by single-crystal XRD, FT-IR, SEM/EDS, TGA/DTA, CHN and UV-visible spectral analysis. Hirshfeld surfaces, derived using single crystal X-ray diffraction data, reveal that the close contacts are associated with strong interactions. Fingerprint plots were used to locate and analyze the percentage of hydrogen bonding interactions.

 Please wait while we load your content...
Please wait while we load your content...