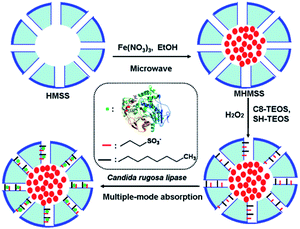
A mixed-function-grafted magnetic mesoporous hollow silica microsphere immobilized lipase strategy for ultrafast transesterification in a solvent-free system†
Mingming Zhenga,
Lijing Maob,
Fenghong Huang*a,
Xia Xianga,
Qianchun Denga and
Yuqi Fengb
aOil Crops Research Institute, Chinese Academy of Agricultural Sciences, Hubei Key Laboratory of Lipid Chemistry and Nutrition, Wuhan 430062, China. E-mail: jiagongzx@oilcrops.cn
bKey Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), Department of Chemistry, Wuhan University, Wuhan 430072, China
First published on 7th May 2015
Abstract
Although enzymatic catalysis is an attractive approach for the green synthesis of chemicals, it often suffers from low reactivity and poor stability during the reaction. In this study, lipase from Candida rugosa (CRL) was immobilized and stabilized on magnetically-separable, mixed-function-grafted, large pore mesostructured magnetic hollow mesoporous silica microspheres (MHMSS) by means of multiple-mode adsorption based on both hydrophobic and strong cation-exchange interactions. Benefiting from the hollow large mesoporous structure, ultrafast enzyme immobilization could be realized in 5 min, with a high loading of CRL (95.2 mg g−1). Stabilized CRL@MHMSS was successfully used for the ultrafast transesterification of phytosterol with fatty acids and triglycerides in a solvent-free system, which reached high conversions (≧90.9%) within 15 min at 55 °C. Magnetic separation of MHMSS facilitated the repeated usage of CRL@MHMSS for more than 50 successive reactions without damaging its catalytic activity. Its high activity and stability make the MHMSS immobilized enzyme an attractive catalyst for green synthesis in a solvent-free system.
1. Introduction
Enzymes are environment-friendly biocatalysts that display high chemo-, stereo-, and regioselectivity while operating under mild conditions.1–3 Thus, enzyme-catalyzed synthesis has attracted increasing attention for large-scale industrial applications in the pharmaceutical and food industries. However, potential problems are the denaturation of native enzymes during the reaction, leading to poor enzyme stability and activity, as well as difficulties in recovery and reuse.4 As an alternative, the use of an immobilized enzyme not only allows enzyme reusability, and hence reduces operational costs, but also reduces enzyme contamination and facilitates easy separation of the products.5,6Among numerous possible carriers, mesoporous materials have attracted considerable attention since they were first reported for potential applications in heterogeneous catalysis, host–guest chemistry, adsorption and drug delivery because of their large specific surface area, tunable porosity, low cytotoxicity, favorable mechanical properties and the ease with which their pore surfaces can be chemically modified.7,8
Hollow mesoporous spheres with mesoporous shells exhibit more advantages in terms of mass diffusion and transportation compared with conventional mesoporous materials due to their larger pores and cavity volumes and spherical morphology.9,10 A number of reports have described the formation of hollow mesoporous silica spheres (HMSS) in the past few years.11–14 However, for the adsorption and immobilization of enzymes, relatively small pore size is one of the major drawbacks limiting their application.15–17 Therefore, it is very important to develop large-pore HMSS composites to improve and extend their application in enzyme immobilization. In addition, incorporating magnetic particles into mesoporous materials led to the development of magnetically-separable and stabilized enzyme systems for facile recycling.18–20 Thus, by combining the texture of hollow mesoporous spheres with magnetic separation properties, magnetic mesoporous spheres would represent a new class of promising carriers for enzyme immobilization.21 Unfortunately, magnetic mesoporous materials with large pore sizes (>10 nm) have seldom been reported to date.17
Adsorption onto solid supports has proven to be an easy and effective way to improve the catalytic performance and stability of enzymes.22 Modification by surface functionalization can increase electrostatic or hydrophobic interactions between support and enzyme molecules, thus decrease the enzyme leaching.23–25 In addition, it has been shown that lipases prefer to adsorb on hydrophobic supports, involving the adsorption of hydrophobic areas surrounding the active center and leaving the active site fully exposed to the reaction medium.26,27 A “multiple-mode” adsorption approach including electrostatic and hydrophobic interactions was employed as an effective means of enzyme immobilization on mesoporous materials.18 This approach not only improves both enzyme loading and activity (especially transesterification activity), but also prevents enzyme denaturation and leaching.25,26 Although lipase-catalyzed esterification and transesterification have been previously reported, most earlier studies were carried out in the presence of organic solvents, which are environmentally unfriendly and prone to resulting in the presence of residual solvents.28 On the contrary, a solvent-free system involves a simple mixture of substrates, offering the advantages of maximization of substrate concentration, greater volumetric production and cost savings in product purification.29
In the present work, lipase from Candida rugosa (CRL) was immobilized on magnetic hollow mesoporous silica spheres (MHMSS) to prepare a magnetically-separable biocatalyst. Solvent-free transesterification. The fabrication strategy and working principle of this composite material are illustrated in Fig. 1. The reusability and thermostability was also dramatically increased after the immobilization. With this immobilization, the fastest transesterification of phytosterol with fatty acids and triglycerides in a solvent-free system was realized.
2. Experimental
2.1 Chemicals and reagents
Sodium silicate nonahydrate (Na2SiO3·9H2O), cetyltrimethylammonium bromide (CTAB), iron nitrate nonahydrate (Fe(NO3)3·9H2O), ethanol (EtOH), ethylene glycol and other chemicals were supplied by Shanghai General Chemical Reagent Factory (Shanghai, China). Purified water was obtained with a Milli-Q apparatus (Millipore, Bedford, MA, USA). Enhanced (BCA) Protein Assay Kit was obtained from Beyotime Institute of Biotechnology.CRL (lyophilized powder, type VII, 700 U mg−1 of solid), and p-nitrophenyl palmitate (p-NPP) were purchased from Sigma-Aldrich. Phytosterols (β-sitosterol (77%), campesterol (17%), stigmasterol (5%)) were purchased from Xian Bluesky Biological Engineering Co. Ltd (Xi'an, China). Linolenic acid (80%), linoleic acid (90%), and oleic acid (90%) were purchased from Henan Linuo Biochemical Co. Ltd (Anyang, China). Refined and bleached algae oil, camellia oil, rapeseed oil, sunflower oil, and linseed oil, which were used as the source of triglycerides, were purchased from the supermarket.
2.2 Preparation of hollow mesoporous silica spheres (HMSS)
HMSS was prepared according to a modified procedure described previously.21 A total of 19.6 g of CTAB followed by 23.2 g of solid Na2SiO3·9H2O were dissolved in 337 mL of water, resulting in clear solution at 30 °C. Afterwards 35 mL of ethyl acetate were quickly added, the mixture was stirred for 30 seconds. Then the mixture was aged at 90 °C for 48 h after allowing standing at 30 °C for 5 h. Finally, the solid product was filtered and washed with purified water and EtOH. The filtered HMSS was dried at room temperature and calcined at 550 °C for 5 h.2.3 Preparation of mixed-mode hollow mesoporous silica spheres with magnetic cores (MHMSS)
MHMSS was prepared according to the procedure reported by Wu et al. with some modification.21 A certain amount of iron nitrate (4.8 g) was dissolved in 200 mL H2O. Then the solution was added to 2.4 g hollow mesoporous silica spheres. The suspension was dried by heating in a microwave about 10 min and then taken out cooling to room temperature and repeated for several times until dry. Subsequently, the product was washed with 50 mL ethanol twice and dried again. The product was impregnated with 1 mL ethylene glycol up to incipient wetness. The impregnated sample was then subjected to heat treatment under nitrogen up to a treatment of 450 °C at the rate of 5 °C min−1 and kept at this temperature for 2 h. The different spheres with introducing different amount of iron nitrate were labeled MHMSS.The octyl and thiol bonded MHMSS were prepared by adding 10 mmol of C8-TEOS, 20 mmol of SH-TEOS, 60 μL triacetate abdominal, 4.0 g of dried MHMSS in 20 mL of anhydrous toluene in a 100 mL hydrothermal reactor, and the mixture was hydrothermal treated at 130 °C for 15 h. The resulting material was filtered through a sintered glass funnel (G3) and then washed with several aliquots of anhydrous ethanol and water in sequence. After being dried overnight at 80 °C under vacuum condition, the octyl and thiol bonded MHMSS were obtained.
For oxidization, the obtained octyl and thiol bonded MHMSS were suspended in H2O2 (30%, w/w for 10 h at room temperature and then washed with water to remove the excess H2O2).25 After being dried overnight at 60 °C under vacuum condition, the mixed-mode MHMSS were obtained.
2.4 Immobilized of CRL on MHMSS
The immobilized lipase CRL was prepared as proposed by Yan et al. with some modification.22 First, 2.0 g of MHMSS was pre-wetted with phosphate buffer (80 mL, 100 mM, pH 5.0). Then immobilization of lipase CRL on the MHMSS via adsorption was studied in phosphate buffer (40 mL, 20 mM, pH 5.0). The initial concentration of lipase was kept at 0.5–7.0 mg mL−1 in buffer solution. The immobilization experiment was conducted at 30 °C for 1–180 min with continuous stirring in vacuum. Consideration of the “pH-memory effect”, the MHMSS were separated from the lipase solution and then washed with buffer solution (20 mM, pH 7.0) three times and then washed with acetone twice. The resulting immobilized lipase (designated CRL@MHMSS) was lyophilized and stored at 4 °C prior to use. The amount of immobilized lipase was obtained by using the equation| Q = [(C0 − C)V]/M | (1) |
2.5 Measurement of the immobilized CRL activity and stability
The enzymatic activities of free and immobilized CRL were measured by the detection of p-nitrophenol, which comes from the hydrolysis of p-NPP. One unit (U) of enzyme activity is defined as the amount of enzyme which catalyzes the production of 1 mmol of p-nitrophenol per minute under the experimental conditions. The relative activity (%) is the ratio between the activity of every sample and the maximum activity of a sample. The activity yield after immobilization is defined by the following equation:| Activity yield (%) = (B × 100)/A | (2) |
The thermal stability assays were performed by the incubation for different times (0–120 min) of CRL@MHMSS and free CRL at 50 °C and 60 °C, respectively. After the enzyme was cooled to room temperature, its activity was measured under standard conditions (pH 7.0, 37 °C) as described above. Residual activities were calculated as the ratio of the activity of the lipase measured after incubation to the maximal activity of the lipase.
2.6 Lipase-catalyzed esterification and transesterification of phytosterols with different acyl donors
Esterification and transesterification condition: phytosterols (3 mmol), fatty acids (oleic acid, linolenic acid or conjugated linoleic acid, 15 mmol) or triglycerides (algae oil, camellia oil, rapeseed oil, linseed oil, sunflower oil, 15 mmol), immobilized CRL (10% of the total substrate weight), molecular sieves 4 Å (20% of the total substrate weight) were added into an Erlenmeyer flask. The solvent had, in advance, been dehydrated with 15% (w/w) molecular sieves 4 Å for at least 24 h. The vials were placed in an air bath shaker at 55 °C and were shaken at 200 rpm for a certain time. Over the time course of the reactions, a portion of the reaction mixture (50 μL) was periodically removed from the reaction for GC analysis. Using FFAs as the acyl donors, the excess FFAs could be removed by washing with ethanol for 5 times, recovered by rotary evaporate and then reused in the next reaction.2.7 Qualitative and quantitative analysis of phytosterol esters
The composition of the crude products was analyzed by GC. An Agilent 6890 series II gas chromatograph (Hewlett-Packard Co., Avondale, PA), equipped with a flame ionization detector (FID) and a fused silica capillary column (DB-5 HT, 15.0 m × 320 μm × 0.10 μm, Agilent Technologies, Palo Alto, CA) was used. The carrier gas was nitrogen, and the total gas flow rate was 3.5 mL min−1. The injector and detector temperatures were maintained at 320 °C and 350 °C, respectively. The oven temperature was held at 210 °C for 2.0 min, then increased to 320 °C at a rate of 10 °C min−1, held at 320 °C for 15 min, then increased to 380 °C at a rate of 10 °C min−1, and finally held at 380 °C for 5 min. The injection volume was 1 μL in split mode. The split ratio was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The conversion of phytosterol esters (%) was calculated from the GC profile of reactants using the following equation:
1. The conversion of phytosterol esters (%) was calculated from the GC profile of reactants using the following equation:
 | (3) |
3. Results and discussions
3.1 Characterization of the MHMSS and CRL@MHMSS
The morphology and structure of the as-synthesized spheres were investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The HMSS exhibit monodisperse, uniform and spherical morphologies with an average diameter of 2.4–3.6 μm (Fig. 2a and b). The hollow structure of the HMSS has been confirmed by the appearance of broken spheres after crushing under high pressure (Fig. 2c). Mesopores (about 10–20 nm) are found throughout the surface of HMSS in the high-resolution SEM images (Fig. 2d). From the TEM images (Fig. 2e), it can be seen that the average diameter of the cavities is about 1.5–2 μm and the wall thickness of the silica layer is about 0.4–0.6 μm. MHMSS retained a super-paramagnetic property with a magnetization value of 21.6 emu g−1 (Fig. S1†), which allows for easy re-dispersion of MHMSS after magnetic capture in a solvent-free enzymatic reaction system (Fig. 2f).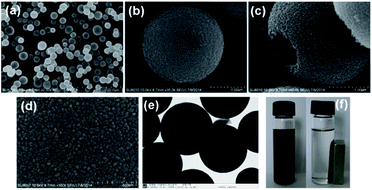 | ||
| Fig. 2 SEM (a)–(d) and TEM (e) images of MHMSS; the dispersion and recovery image of the CRL@MHMSS in a magnetic field (f). | ||
The N2 adsorption–desorption isotherms and pore size distributions of HMSS, MHMSS and CRL@MHMSS were measured (Fig. S2†). N2 sorption isotherms of HMSS and MHMSS (Fig. S2a†) exhibited a typical type IV curve with a H1-type hysteresis loop at a relative pressure (p/p0) range of 0.4–1.0, suggesting the formation of mesopores. As listed in Table S1,† the surface area decreased gradually in the order: HMSS > MHMSS > CRL@MHMSS; and the pore volume also decreased in the same order. Compared with HMSS, the pore volumes of MHMSS and CRL@MHMSS decreased sharply, while the pore size distribution showed little change (Fig. S2b†). This indicated that most of the magnetic particles had been introduced into the cavities of HMSS, and moreover, that CRL had been immobilized in the mesopores of the MHMSS. Correspondingly, the mesopore size calculated from the adsorption branch using the Barrett–Joyner–Halenda (BJH) method (Fig. S2b†) reveals a narrow distribution centered at 16 nm. Moreover, it also shows that the MHMSS composites possess many micropores, a high surface area (380 m2 g−1) and a large pore volume (1.19 cm3 g−1).
To confirm that lipase from Candida rugosa was immobilized on the MHMSS, the FT-IR spectra of MHMSS, CRL@MHMSS and native lipase were investigated (Fig. S3†). For MHMSS, the bands at 804 and 1089 cm−1 could be assigned to the symmetric stretching vibration of Si–O and antisymmetric stretching vibration of Si–O–Si.30 For the native lipase, the peaks observed in the regions 1410 and 1539 cm−1 corresponded to the bending vibrations of –C–H and –N–H.31 The –C–H and –N–H absorption peaks observed in the FT-IR spectrum of CRL@MHMSS confirmed that lipase from Candida rugosa had been successfully immobilized on MHMSS.
3.2 Lipase immobilization on the MHMSS carriers with ultrahigh speed
It is crucial that the adsorption of enzyme be controllable for the successful preparation of immobilized enzyme. For comparison with the MHMSS immobilized lipase, the silica beads (3 μm size, 280 m2 g−1, 10 nm pore size) functionalized with octyl and sulfonic acid groups with the same way were chosen as a host material. Lipase from Candida rugosa (60 kDa) was immobilized on silica beads (SBs) and MHMSS by multiple-mode adsorption (the immobilized lipases were named CRL@SBs and CRL@MHMSS, respectively). The loading amounts and loading times of the lipase on the different carriers were obtained and are summarized in Fig. 3. These two carriers adsorbed CRL well, and the adsorption amounts and equilibrium times were measured to be 59.8 mg g−1 and 60 min for irregular silica and 95.2 mg g−1 and 5 min for MHMSS. The amount of protein immobilized and the equilibrium time on MHMSS were 1.6-fold larger and 12-fold shorter than those on SBs, indicating that MHMSS with larger pore sizes and higher surface areas and pore volumes are much better candidates for adsorption immobilization of enzymes. The theoretically loading amount was calculated base on the dimension of lipase and the pore volume of the MHMSS carriers and was about 217.3 mg g−1.The effect of initial protein concentration on the protein amount fixed in the carrier and the specific activity was illustrated by Fig. S4.† The protein amount in the carrier increased to 96.09 mg g−1 as the initial protein amount increased to 4.7 mg mL−1. Furthermore, the specific activity also reached the equilibrium as the protein concentration further increased. The maximum specific activity for p-NPP hydrolysis was 432 U g−1 protein.
3.3 Thermal stability of free CRL and CRL@MHMSS
Thermal stability experiments were carried out with samples of free and immobilized CRL, which were incubated in the absence of substrate at 50 °C and 60 °C, respectively. As shown in Fig. 4, the activity of immobilized CRL decreased less and more slowly than the free form at both temperatures. Free CRL had lost half of its activity within only 40 min at 50 °C. In contrast, CRL@MHMSS still retained 69.8% of its initial activity after 120 min of heat treatment at 50 °C. This phenomenon was more obvious at higher temperature (60 °C), proving the enhanced thermostability of this composite material. This is attributed to the stabilizing effect of the MHMSS matrix with its large mesopores and the hydrophobic and cation-exchange interactions between CRL and carrier, which prevent the extensive conformational changes typical of thermal denaturation.20 The ability to retain enzyme activity at high temperatures affords a number of advantages such as improved substrate solubility, reaction rate and conversion, thereby expanding the range of applications of enzymatic synthesis.3.4 Ultrafast transesterification of phytosterol with fatty acids and triglyceride
The effect of hydrophobicity on the activity of the immobilized lipase was investigated. Three kinds of MHMSS materials functionalized with pure hydrophobic (C8), pure cation exchange (SO3) and multiple-mode groups were prepared and used as the carriers for lipase immobilization, respectively. As shown in Table 1, The SO3-MHMSS showed lowest hydrophobicity and exhibited the lowest apparent activity. The C8-MHMSS showed the highest hydrophobicity and exhibited the higher apparent activity than SO3-MHMSS. The result showed that lipases easy to exhibit high activity when adsorb on hydrophobic supports, involving the adsorption of hydrophobic areas surrounding the active center and leaving the active site fully exposed to the reaction medium.32–34 However, the C8-MHMSS was very difficult to suspend in an aqueous lipase solution; furthermore, lipase solution is difficult to access the inner surface of the pores because of the highly hydrophobic surfaces, leading to the lowest immobilized lipase amount.33 The multiple-mode MHMSS possessing both hydrophobic and cation exchange groups showed the moderate hydrophobicity and exhibited the highest immobilized amount and apparent activity among the three carriers.| Functional groups | Contract angle (°) | Immobilized amount (mg g−1) | Apparent activity (μmol g−1 min−1) |
|---|---|---|---|
| C8 | 117.2 | 35.4 | 80.2 |
| Multiple-mode | 81.5 | 95.2 | 350.1 |
| SO3 | 10.2 | 54.5 | 30.4 |
The transesterification of phytosterol with fatty acids and triglycerides was used as the model reaction to investigate the catalytic performance of CRL@MHMSS in a solvent-free system. Table 2 summarizes the data on conversion for the esterification and transesterification of phytosterols with different acyl donors including free fatty acids (FFAs) and triglycerides. As listed in Table 2, the conversions were above 90.9% and 92.1% for FFAs and triglycerides (five kinds of edible oils), respectively, in a solvent-free system. The maximum apparent activity and specific activity of CRL@MHMSS were 350.1 μmol g−1 CRL@MHMSS per min and 3688.1 μmol g−1 protein per min, respectively, which was about 3 times higher than the finding of No et al.28
| Acyl donors | Time (min) | Conversion (%) | |
|---|---|---|---|
| Fatty acids | Oleic acid | 15 | 90.9 |
| Linoleic acid | 92.6 | ||
| Linolenic acid | 95.3 | ||
| Triglycerides | Algae oil | 15 | 92.7 |
| Camellia oil | 94.3 | ||
| Rapeseed oil | 95.8 | ||
| Linseed oil | 92.1 | ||
| Sunflower oil | 96.3 | ||
These results suggested that the immobilized lipase CRL@MHMSS demonstrated high catalytic activity and the corresponding transesterifications of phytosterol with different kinds of acyl donors all reached relatively high conversions (above 90.9%). As far as we know, the times for phytosterol ester formation with a conversion of above 90% are the shortest with both fatty acids and triglycerides as the acyl donors reported to date. Using FFAs as the acyl donors, the excess FFAs could be recovered and reused in the next reaction; using triglyceride as the acyl donors, the functional oil rich in PEs could be obtained without purification. In a word, the whole process is almost no by-products and waste generation. Furthermore, enzymatic processes conducted in a solvent-free system provide the further advantages of food safety, operation simplicity and decreased environmental hazards.29
The reusability of the immobilized enzyme is rather important for practical applications. At the end of each reaction batch, the CRL@MHMSS was washed with hexane to remove any substrate and product retained on the support and then lyophilized. The immobilized CRL was reused after each reaction cycle. The effect of repeated CRL@MHMSS and CRL@SBs use on the conversion of phytostanyl linolenate in a solvent-free system was investigated (Fig. 5). It was observed that CRL@MHMSS maintained excellent activity after 50 reuses and the conversion of phytostanyl linolenate remained at 92.6%, whereas CRL@SBs lost most of its activity (33.0%) after only 10 cycles. This result confirmed that the immobilized CRL on MHMSS allowed not only excellent activity in a solvent-free system, but also satisfactory reusability over 50 reaction cycles. Benefiting from the large mesopores (10–20 nm) of the MHMSS matrix, the excellent enzymatic activity and good reproducibility can be attributed to better mass transfer of substrates and products in a high viscosity solvent-free system and the robust immobilization of MHMSS that did not affect the active sites and subunits, respectively. Firstly, the MHMSS could protect the lipase from mechanical inactivation caused by shaking. Secondly, the multiple-mode adsorption stabilized the open-form of the lipases and simultaneously inhibited lipase leakage and denaturation effectively.26 Furthermore, CRL@MHMSS could be recovered easily by magnetic capture compared with CRL@SBs and native lipase, which could reduce the loss of lipase during the recycling process. The advantages mentioned above make it possible for CRL@MHMSS to be applied as an efficient biocatalyst on an industrial scale. Such high reusability (50 reuses) of immobilized enzyme with relatively high conversion in a solvent-free catalyst system has seldom been reported.
 | ||
| Fig. 5 Comparison of recyclability of CRL@MHMSS and CRL@SBs in the transesterification of phytosterol with linolenic acid in a solvent-free system. | ||
4. Conclusions
In conclusion, MHMSS with large surface areas, high pore volumes and large pore diameters were employed as hosts for lipase from Candida rugosa. The adsorption of CRL in the pores of MHMSS resulted in a highly stable and active magnetic multiple-mode immobilized enzyme. Compared with CRL@SBs, CRL@MHMSS exhibited excellent thermal stability and catalytic activity in a solvent-free system. When CRL@MHMSS was used as a biocatalyst for transesterification reactions, improved activity and reusability were achieved. With these desired characteristics, this approach of enzyme adsorption on MHMSS materials could be employed to realize the immobilization of other enzymes with improved stability and catalytic activity, and may have potential applications in various enzyme-based industrial processes.Acknowledgements
This work was supported by the National Natural Science Foundation of China (31371843, 3110353), the youth talents development program of Hubei province, the Earmarked Fund for China Agriculture Research System (CARS-13) and the Director Fund of Oil Crops Research Institute (1610172014006).Notes and references
- R. Wang, Y. Zhang, J. Huang, D. Lu, J. Ge and Z. Liu, Green Chem., 2013, 15, 1155–1158 RSC.
- R. A. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS PubMed.
- U. Hanefeld, L. Gardossi and E. Magner, Chem. Soc. Rev., 2009, 38, 453–468 RSC.
- Y. Kuwahara, T. Yamanishi, T. Kamegawa, K. Mori, M. Cheb and H. Yamashita, Chem. Commun., 2012, 48, 2882–2884 RSC.
- R. A. Sheldon and S. Van Pelt, Chem. Soc. Rev., 2013, 42, 6223–6235 RSC.
- R. C. Rodrigues, C. Ortiz, A. Berenguer-Murcia, R. Torres and R. Fernández-Lafuente, Chem. Soc. Rev., 2013, 42, 6290–6307 RSC.
- M. Hartmann and X. Kostrov, Chem. Soc. Rev., 2013, 42, 6277–6289 RSC.
- V. Gascón, I. Díaz, R. M. Blanco and C. Márquez-Álvarez, RSC Adv., 2014, 4, 34356–34368 RSC.
- Y. Li, J. Shi, Z. Hua, H. Chen, M. Ruan and D. Yan, Nano Lett., 2003, 3, 609–612 CrossRef CAS.
- J. Liu, W. Wang, H. Liu, Y. Zhou, H. Zhang and X. Zhou, RSC Adv., 2014, 4, 25983–25992 RSC.
- L. M. Guo, J. T. Li, L. X. Zhang, J. B. Li, Y. S. Li, C. C. Yu, J. L. Shi, M. L. Ruan and J. W. Feng, J. Mater. Chem., 2008, 18, 2733–2738 RSC.
- Y. Wan and D. Y. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed.
- I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS PubMed.
- M. Vallet-Regí, F. Balas and D. Acros, Angew. Chem., Int. Ed., 2007, 46, 7548–7558 CrossRef PubMed.
- Y. H. Deng, D. W. Qi, C. H. Deng, X. M. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS PubMed.
- W. Li and D. Y. Zhao, Adv. Mater., 2013, 25, 142–149 CrossRef CAS PubMed.
- J. P. Yang, F. Zhang, W. Li, D. Gu, D. K. Shen, J. W. Fan, W. X. Zhang and D. Y. Zhao, Chem. Commun., 2014, 50, 713–715 RSC.
- E. T. Hwang, B. Lee, M. L. Zhang, S. H. Jun, J. M. Shim, J. Lee, J. Kim and M. B. Gu, Green Chem., 2012, 14, 1884–1887 RSC.
- M. Kalantari, M. Kazemeini, F. Tabandeha and A. Arpanaei, J. Mater. Chem., 2012, 22, 8385–8393 RSC.
- T. Hyeon, J. Lee, H. Bin Na, B. C. Kim, J. H. Lee, B. Lee, J. H. Kwak, Y. Hwang, J. G. Park, M. B. Gu, J. Kim, J. Joo, C. H. Shin, J. W. Grate and J. Kim, J. Mater. Chem., 2009, 19, 7864–7870 RSC.
- J. H. Wu, X. S. Li, Y. Zhao, Q. Gao, L. Guo and Y. Q. Feng, Chem. Commun., 2010, 46, 9031–9033 RSC.
- Z. Boros, D. Weiser, M. Márkus, E. Abaháziová, Á. Magyar, A. Tomin, B. Koczka, P. Kovács and L. Poppe, Process Biochem., 2013, 48, 1039–1047 CrossRef CAS PubMed.
- A. Galarneau, M. Mureseanu, S. Atger, G. Renard and F. Fajula, New J. Chem., 2006, 30, 562–571 RSC.
- A. Popat, S. B. Hartono, F. Stahr, J. Liu, S. Z. Qiao and G. Q. Lu, Nanoscale, 2011, 3, 2801–2818 RSC.
- M. M. Zheng, Y. Lu, L. Dong, P. M. Guo, Q. C. Deng, W. L. Li, Y. Q. Feng and F. H. Huang, Bioresour. Technol., 2012, 115, 141–146 CrossRef CAS PubMed.
- N. Rueda, J. C. S. dos Santos, R. Torres, C. Ortiz, O. Barbosa and R. Fernandez-Lafuente, RSC Adv., 2015, 5, 11212–11222 RSC.
- E. A. Manoel, J. C. S. dos Santos, D. M. G. Freire, N. Rueda and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2015, 71, 53–57 CrossRef CAS PubMed.
- S. de No, T. Zhao, J. Lee, J. S. Lee and I. H. Kim, J. Agric. Food Chem., 2013, 61, 8934–8940 CrossRef PubMed.
- C. H. Kuo, T. A. Liu, J. H. Chen, C. M. J. Chang and C. J. Shieh, Biocatal. Agric. Biotechnol., 2014, 3, 1–6 Search PubMed.
- L. Y. Xia, M. Q. Zhang, C. E. Yuan and M. Z. Rong, J. Mater. Chem., 2011, 21, 9020–9026 RSC.
- L. Zhu, X. Y. Liu, T. Chen, Z. G. Xu and W. F. Yan, Appl. Surf. Sci., 2012, 258, 7126–7134 CrossRef CAS PubMed.
- C. Bernal, A. Illanes and L. Wilson, Langmuir, 2014, 30, 3557–3566 CrossRef CAS PubMed.
- C. Garcia-Galan, A. Berenguer-Murcia, R. Fernandez-Lafuente and R. C. Rodrigues, Adv. Synth. Catal., 2011, 353, 2885–2904 CrossRef CAS PubMed.
- E. Magner, Chem. Soc. Rev., 2013, 42(15), 6213–6222 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of the immobilized lipase. See DOI: 10.1039/c5ra05611j |
| This journal is © The Royal Society of Chemistry 2015 |