(2-Chlorobenzoyloxy)copper(i) catalyzed C–S cross-coupling of di(hetero)aryl disulfides with aryl boronic acids under base-free conditions†
Abstract
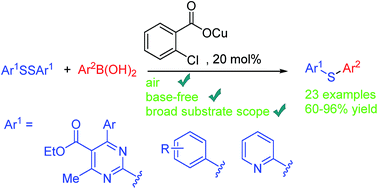
(2-Chlorobenzoyloxy)copper(I) exhibited high activity towards the challenging C–S cross-coupling of di(hetero)aryl disulfides with aryl boronic acids. Herein, diheteroaryl as well as diaryl thioethers were produced with good to excellent yields under base-free conditions.

 Please wait while we load your content...
Please wait while we load your content...