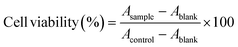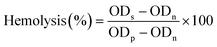Multifunctional hybrid nanoparticles based on sodium carboxymethylcellulose-graft-histidine and TPGS for enhanced effect of docetaxel†
Weihua Jianga,
Lei Yanga,
Lipeng Qiub,
Jingwen Xuc,
Xiuchun Yanga,
Ju Wanga,
Hui Zhoud and
Dongkai Wang*a
aDepartment of Pharmaceutics, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China. E-mail: wangycsyphu@126.com; Fax: +86 24 23986310; Tel: +86 24 23986310
bDepartment of Pharmaceutics, School of Pharmaceutical Sciences, Jiangnan University, Wuxi 214122, P. R. China
cDepartment of Pharmacology, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China
dPharmaceutical Department, The First Hospital of China Medical University, Shenyang, 110001, P. R. China
First published on 10th June 2015
Abstract
Multifunctional hybrid nanoparticles (NPs) based on sodium carboxymethylcellulose-graft-histidine (CMH) and TPGS were designed for effective delivery and overcoming multidrug resistance (MDR) of docetaxel (DTX). An amphiphilic CMH copolymer with excellent biocompatibility, pH-sensitivity, and solubilizing capacity for poorly soluble drugs was firstly synthesized and characterized. TPGS incorporated into CMH copolymer facilitated lower critical aggregation concentration (CAC) (0.0074 mg mL−1), smaller particle size (147.8 ± 3.14 nm), higher drug encapsulation (88.3 ± 3.33%), and better controlled release rate. The NPs based on CMH copolymer showed a pH-dependent drug release manner. The DTX-loaded CHT-1.5 NPs could significantly enhance cytotoxicity against both MCF-7 and MCF-7/ADR cells when compared to DTX-loaded CMH NPs and free DTX. Furthermore, cellular uptake and P-gp inhibition assays confirmed that the enhanced MDR reversal effect of DTX-loaded CHT-1.5 NPs was caused by the combination of increased drug accumulation and inhibition of P-glycoprotein mediated drug efflux in drug-resistant MCF-7/ADR cells. Consequently, CHT-1.5 NPs can act as an effective nanocarrier for delivering antitumor drugs and overcoming P-gp overexpressing tumor cells.
1 Introduction
Docetaxel (DTX) belonging to the taxane family of antimitotic agents is commonly used in the treatment of various types of solid tumors including ovarian, non-small cell lung, breast, and head and neck cancer.1 Due to its low aqueous solubility, the current formulation Taxotere® is prepared with high concentration of Tween 80, which is known to cause severe allergic reactions and peripheral neuropathology.2 Furthermore, multidrug resistance (MDR) to DTX has also limited its potential application and overall clinical utility. The most common underlying mechanism of drug resistance is over-expression of the multidrug efflux pump P-glycoprotein (P-gp), which is a member of the large ATP-binding cassette (ABC) transporter family.3,4 Membrane-bound P-gp can actively extrude DTX, thus lowering the accumulation of drugs within the cells.5 To overcome the disadvantages of the commercial formulation and increase the therapeutic effect of DTX, much effort has been devoted to new delivery vesicles using proper materials. Self-assembled nanoparticles (NPs) based on amphiphilic copolymers have aroused considerable attention due to their easy preparation, small size, and tumor targeting via the enhanced permeability and retention (EPR) effect.6 More importantly, the hydrophobic microdomain of its unique core–shell structure could incorporate and solubilize poorly soluble drugs effectively. In addition, they may circumvent P-gp efflux by endocytosis.5 However, the free intracellular drugs were still extruded by efflux system because many nanocarriers themselves could not inhibit the P-gp function.7,8 As we know an ideal vehicle is not only designed for hydrophobic drug delivery but also expected to increase the antitumor efficiency of agents. Hybrid nanocarriers by incorporating P-gp blocking surfactants into polymeric NPs highlight significant advantages. On one hand, the hybrid NPs could inhibit the effect of P-gp to overwhelm MDR.9 On the other hand, the drug loading efficiency, colloidal stability, and drug release profile could be effectively modulated by alteration of the type and ratio of copolymers as compared to single copolymer NPs.10,11Based on above rationales that drug carriers should be safe and effective, we aim to develop a multi-functional hybrid nanoparticle system by incorporating TPGS into a pH-sensitive sodium carboxymethylcellulose-graft-histidine (CMH) copolymer. Sodium carboxymethylcellulose (CMC) is one of the most important excipients in pharmaceutical products (FDA inactive ingredients database) due to its high stability, non-toxicity, biodegradability and biocompatibility. CMC-based nanocarriers showed promising applications in drug delivery field.12,13 For example, the PEGylated carboxymethylcellulose conjugate of docetaxel was demonstrated to improve the pharmacokinetics, biodistribution and anticancer efficacy of DTX compared to Taxotere®.14–16 With pH-sensitivity and much perfect biocompatibility, the histidine moiety has been suggested to be introduced into polymers as side-chain pendants to design amphiphilic copolymers.17 Moreover, the most remarkable advantage of histidine is the “proton sponge effect” caused by the protonation of imidazole group, which could disrupt the lysosomal membrane and facilitate the rapid anticancer drug release in the cytoplasm.18 Therefore, we combined the characteristics and advantages of the two materials to synthesize a novel pH-sensitive CMH copolymer for drug delivery.
D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS), a water-soluble derivative of natural vitamin E, comprised of a lipophilic alkyl tail and a hydrophilic polar head portion. Nanopreparations based on TPGS have been proved to increase drug solubility, improve the colloid stability, enhance cellular uptake, and inhibit P-gp mediated MDR.19,20 TPGS is an effective anti-cancer substance. It could induce apoptosis in cancer cells without affecting normal cells21 and synergistically with chemotherapeutic drugs to increase the efficacy of nanoparticle formulations.22 Unfortunately, TPGS itself can hardly be used to carry anticancer drugs due to the relatively high critical aggregation concentration (CAC, 0.2 mg mL−1).21 Thankfully, due to its relatively bulky lipophilic portion, it is an ideal component material to form hybrid NPs.23,24
In this study, we intended to concentrate the advantages of CMH and TPGS polymers on one nanovehicle. The multifunctional hybrid NPs are expected to passively accumulate in the tumor tissue by EPR effect. After internalized into drug-resistant cancer cells via endocytosis, the hybrid NPs disassemble in the acidic lysosomes, release DTX and TPGS into the cytosol to suppress P-gp mediated efflux, and thus achieve the MDR reversal effect (Scheme 1). More specifically, CMH copolymer was synthesized by EDC/NHS-mediated coupling reaction and characterized in detail. The effect of TPGS segment on the physicochemical and pharmaceutical properties of CMH/TPGS (CHT) NPs was further investigated. The cellular uptake and cytotoxicity of DTX-loaded NPs against MCF-7 and MCF-7/ADR cells were examined. The effect of CHT-1.5 NPs on the P-gp activity was measured by flow cytometer using rhodamine-123 as a substrate of P-gp.
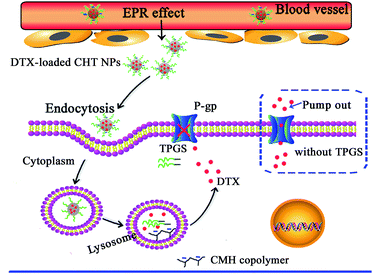 | ||
| Scheme 1 Representation of the accumulation of DTX-loaded CHT NPs in tumor tissue by the EPR effect, endocytosis, and the inhibition of P-gp mediated efflux system. | ||
2 Materials and methods
2.1 Materials
Sodium carboxymethylcellulose low viscosity (degree of substitution (DS) = 0.81) was kindly provided by Sunhere Pharmaceutical Excipients Co., Ltd (China). L-Histidine (histidine) was obtained from Shanghai Kyowa Amino Acid Co., Ltd (Shanghai, China). D-α-Tocopheryl polyethylene glycol 1000 succinate was kindly supported by Beijing Fengli Jingqiu Commerce and Trade Co., Ltd. Ethyl-3-(3-dimethylaminoprop-yl)-carbodiimide HCl (EDC), N-hydroxysuccinimide (NHS) and rhodamine-123 (Rh123) were purchased from Aladdin. 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Beyotime (Shanghai, China). Pyrene, coumarin-6 (C6), and 4-dimethylaminopyridine (DMAP) were obtained from J&K Scientific Ltd. Docetaxel was purchased from Beijing Norzer Pharmaceutical Co., Ltd. All other reagents were analytical grade and used without any further purification.2.2 Cell culture
The human breast cancer MCF-7 cell line and MCF-7/ADR cell line were provided by Chinese Academy of Sciences (Shanghai, China) and KeyGen Biotech Co. Ltd (Nanjing, China) respectively. The mouse L929 fibroblasts cell line was purchased from American Type Culture Collection (ATCC Distributor Beijing Zhongyuan Ltd, Beijing, China). L929 and MCF-7 cells were cultured using DMEM supplemented with 10% (v/v) FBS and 1% (v/v) penicillin (100 U mL−1) and streptomycin (100 μg mL−1). MCF-7/ADR cells were cultured with RPMI 1640 medium with 10% (v/v) FBS and 1% (v/v) penicillin (100 U mL−1) and streptomycin (100 μg mL−1) and 1000 ng mL−1 doxorubicin. Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere.2.3 Synthesis and characterization of pH-sensitive CMH copolymer
Firstly, the hydroxyl groups of CMC were acetylated with acetic anhydride in order to generate a solvent-soluble cellulose derivative according to the method from literature.14 Then, histidine moiety was coupled to the acetylated CMC (CMC-Ac) by EDC/NHS-mediated coupling reaction. CMC-Ac (0.150 g, 0.6 mmol carboxyl) was dissolved in MeCN (1 mL). EDC (0.72 mmol) was dissolved in a mixture of MeCN (6 mL) and distilled water (0.3 mL). NHS (0.72 mmol) and DMAP (0.06 mmol) were dissolved in MeCN (1 mL). Histidine (0.467 g, 3.0 mmol) was dissolved in distilled water. Then EDC, NHS, and DMAP reagents were added to the CMC-Ac solution, followed by addition of histidine. The mixed solution was stirred for 48 h at room temperature, and then dialyzed (molecular weight cut-off 8000 Da, Greenbird Inc., Shanghai, China) for 3 days against distilled water. The final product was dried by lyophilization. The chemical structure of CMH was determined by proton nuclear magnetic resonance (1H NMR, Bruker 600, Switzerland) using DMSO as the solvent.Cytotoxicity of CMH copolymer against L929 cells was monitored using the MTT assay. All cells at a logarithmic growth phase, were detached and plated in 96-well microplates (Costar, IL, USA) at densities of 5 × 103 cells per well. The cells were then exposed to various concentrations of CMH solution. After 24 h incubation, 20 μL MTT was added to the medium and further incubated for 4 h. The medium in each well was removed and 100 μL DMSO was added to dissolve the internalized purple formazan crystals. The absorbance was monitored at 490 nm in a Thermo Scientific microplate reader (Multiskan GO, Finland). Cell viability was calculated by the following equation:
Level of hemolysis of CMH copolymer was investigated using red blood cells (RBCs).6 Tween 80 was selected as the control. After collection, the rabbit RBCs were centrifuged at 1500 rpm for 10 min and washed three times with normal saline to harvest the erythrocytes. The concentration of the final washed erythrocytes suspended was 2% (v/v) and stored at 4 °C. The copolymer and Tween 80 were diluted with normal saline to different concentrations ranging from 0.1 to 3.0 mg mL−1. Each sample solution was mixed with the same volume of 2% v/v RBC suspension. The mixture was incubated at 37 °C for 2 h and then centrifuged at 3000 rpm for 10 min. The absorbance at 545 nm of the supernatant was measured by a UV-vis spectrophotometer (UV-9100, Beijing Rayleigh Analytical Instrument Corporation). Hemolysis of each sample was calculated with the formulation as follows:
The pH-responsive behavior of CMH was confirmed by the light transmittance. CMH copolymer was dissolved in PBS at pH 8.0 to obtain a concentration of 2.0 mg mL−1. Then, 5.0 mL of the CMH solution was taken and its pH was adjusted from pH 8.0 to 3.5 with hydrochloric acid and the changes in transmittance were measured with the UV-vis spectrometer at a wavelength of 500 nm.
2.4 Determination the solubilizing effect of CMH on DTX
The solubility of DTX in CMH solution was measured by adding excess amount of DTX into 1 mL of phosphate-buffered saline (PBS, pH 7.4) containing 0.1%, 0.2%, 0.3%, or 0.5% (w/v) CMH in the centrifugal tube, followed by intensely shaking at 25 °C for 48 h. Tween 80 served as the control. Then the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and filtered through a 0.45 μm microfiltration membrane, after which the drug concentration in each vehicle was determined by high performance liquid chromatography (HPLC, LC-10AT, Shimadzu, Japan) equipped with a reverse phase C-18 column (Diamonsil (2), 250 × 4.6 mm, 5 μm).25 The mobile phase consisted of MeCN and deionized water (54/46, v/v) with a flow rate at 1 mL min−1. The detection wavelength was set at 228 nm.
000 rpm for 10 min and filtered through a 0.45 μm microfiltration membrane, after which the drug concentration in each vehicle was determined by high performance liquid chromatography (HPLC, LC-10AT, Shimadzu, Japan) equipped with a reverse phase C-18 column (Diamonsil (2), 250 × 4.6 mm, 5 μm).25 The mobile phase consisted of MeCN and deionized water (54/46, v/v) with a flow rate at 1 mL min−1. The detection wavelength was set at 228 nm.
2.5 Self-aggregation behaviors of “pure” CMH copolymer and CMH/TPGS mixtures
The CAC values of CMH copolymer and the mixtures composed of CMH and TPGS (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 3
1.5, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) were determined by fluorescence measurement using pyrene as a probe.26 Aliquots of pyrene stock solution (5.0 × 10−6 M in acetone, 100 μL) were transferred into a series of volumetric flasks, and the acetone was evaporated. A series of samples dissolved in PBS (pH 7.4) with concentrations ranging from 0.005 to 1 mg mL−1 were added into each flask to achieve a final pyrene concentration of 5.0 × 10−7 M. The combined solution was equilibrated at room temperature in the dark for 24 h. The fluorescence spectra were obtained using a microplate reader (BIO-RAD 680, American) at 25 °C. The excitation spectra were scanned from 300 to 350 nm with the emission wavelength of 385 nm. The first point of inflection of intensity ratio (I338/I335) against log polymer concentration was taken as CAC value.
2 w/w) were determined by fluorescence measurement using pyrene as a probe.26 Aliquots of pyrene stock solution (5.0 × 10−6 M in acetone, 100 μL) were transferred into a series of volumetric flasks, and the acetone was evaporated. A series of samples dissolved in PBS (pH 7.4) with concentrations ranging from 0.005 to 1 mg mL−1 were added into each flask to achieve a final pyrene concentration of 5.0 × 10−7 M. The combined solution was equilibrated at room temperature in the dark for 24 h. The fluorescence spectra were obtained using a microplate reader (BIO-RAD 680, American) at 25 °C. The excitation spectra were scanned from 300 to 350 nm with the emission wavelength of 385 nm. The first point of inflection of intensity ratio (I338/I335) against log polymer concentration was taken as CAC value.
2.6 Preparation and characterization of DTX-loaded NPs
DTX-loaded NPs were prepared by the solvent evaporation-induced self-assembly method. Briefly, 15 mg of CMH and different doses of TPGS (0, 5, 7.5, and 10 mg) were dissolved in 3 mL of PBS 7.4. Then 1 mg of DTX dissolved in 2 mL of MeCN was added to the above solution. After 2 h of stirring at room temperature, MeCN was removed by the rotary evaporation. Unloaded DTX was removed by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Finally, the DTX-loaded NPs were filtered with a microfiltration membrane (0.45 μm). Blank CHT NPs were prepared by the same procedure. The size was determined by dynamic light scattering (DLS, Zetasizer Nano-ZS90, Malvern Instruments Ltd, Malvern, UK). The zeta potential was determined by laser doppler velocimetry (LDV) using the same instrument. The morphology of DTX-loaded CHT-1.5 (CMH/TPGS 3
000 rpm for 10 min. Finally, the DTX-loaded NPs were filtered with a microfiltration membrane (0.45 μm). Blank CHT NPs were prepared by the same procedure. The size was determined by dynamic light scattering (DLS, Zetasizer Nano-ZS90, Malvern Instruments Ltd, Malvern, UK). The zeta potential was determined by laser doppler velocimetry (LDV) using the same instrument. The morphology of DTX-loaded CHT-1.5 (CMH/TPGS 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) NPs was observed by scanning electron microscope (SEM, Hitachi S-3400N, Japan) and transmission electron microscopy (TEM, JEM-1200 EX, Japan). SEM was operated at an accelerating voltage of 5 kV. Prior to imaging, a small quantity of freeze-dried sample powders were fixed on the metal stubs and sputtered with gold under vacuum for 30 s. TEM was performed at an acceleration voltage of 80 kV. A drop of the nanoparticle solution was placed on the copper grids coated with carbon film and stained with phosphotungstic acid (2%, w/w) for 2 min before testing.
1.5) NPs was observed by scanning electron microscope (SEM, Hitachi S-3400N, Japan) and transmission electron microscopy (TEM, JEM-1200 EX, Japan). SEM was operated at an accelerating voltage of 5 kV. Prior to imaging, a small quantity of freeze-dried sample powders were fixed on the metal stubs and sputtered with gold under vacuum for 30 s. TEM was performed at an acceleration voltage of 80 kV. A drop of the nanoparticle solution was placed on the copper grids coated with carbon film and stained with phosphotungstic acid (2%, w/w) for 2 min before testing.
The physical state of DTX in CHT-1.5 NPs was measured by the differential scanning calorimetry (DSC, DSC-60, Shimadzu, Japan) and powder X-ray diffraction (PXRD, D/max 2500PC, Rigaku, Japan). For the analysis of DSC, the measurement was carried out in the temperature range of 30–300 °C under nitrogen at a scanning rate of 10 °C min−1. The analysis of PXRD was determined between 3° and 50° (2θ angle range). The scan time and step size were 10 s and 0.02°, respectively.
2.7 DTX loading efficiency and encapsulation efficiency
The drug loading (DL) and encapsulation efficiency (EE) were also evaluated. Briefly, 0.5 mL DTX-loaded nanoparticle solution was disrupted in 9.5 mL methanol and sonicated for 5 min. After filtered through a 0.45 μm microfiltration membrane, the concentration of DTX was determined by HPLC. The DL and EE were calculated as the ratio of the drug weight in the NPs to the total weight of the NPs and feeding weight of drug, respectively.2.8 In vitro drug release study
Aliquots of DTX-loaded nanoparticle solution were placed in a dialysis bag with a molecular weight cut-off of 3.5 kDa. The dialysis bag was immersed in 50 mL acetate buffer (pH 4.5) or PBS (pH 7.4) containing Tween 80 (0.5% w/v) at 37 °C with gentle shaking. At predetermined time intervals, 1.5 mL of the incubation medium was removed, while an equivalent volume of fresh medium was added. The amount of DTX released at each time point was determined by HPLC. In vitro release profile was obtained by representing the percentage of drug released with respect to the amount of DTX encapsulated in the NPs.2.9 In vitro analysis of DTX cytotoxicity
Cytotoxicity against MCF-7 and MCF-7/ADR cells was monitored using the MTT assay. Cells were seeded in 96-well plates at 5 × 103 cells per well and incubated for 24 h. The medium was then replaced by CMH copolymer, blank CHT-1.5 NPs, free DTX (dissolved in DMSO), DTX-loaded CHT-1.5 NPs, and DTX-loaded CMH NPs at various drug concentrations. The concentration of CMH copolymer and blank CHT-1.5 NPs in the cell culture was matched to the drug concentration. After 48 h incubation, the medium was removed and the wells were washed three times with PBS. MTT assay was used to measure the cell viability according to the method described in Section 2.3. The 50% growth inhibition (IC50) values of different groups were then calculated. The resistant index (RI) and reversal factor (RF) were calculated as the following formulations to evaluate the MDR reversal effect of the NPs.| RI = IC50(MCF-7/ADR)/IC50(MCF-7) |
| RF = IC50(free DTX)/IC50(DTX-loaded NPs) |
2.10 In vitro cellular uptake studies
The cellular uptake efficiency of the developed CHT-1.5 NPs was evaluated by CLSM using coumarin-6 (C6) as a fluorescence probe.27 C6-loaded CMH NPs and CHT-1.5 NPs were similarly prepared except that DTX was replaced by C6. MCF-7 and MCF-7/ADR cells were seeded into 6-well black plates at 3 × 105 cells per well and after the cells reached 80% confluence, the C6-loaded CMH NPs, C6-loaded CHT-1.5 NPs, and free C6 (DMSO solution) dispersed in the cell culture medium (C6 content: 200 ng mL−1) were added into the wells. After incubation for 2 h, cells were washed three times and then fixed with 4% paraformaldehyde (v/v) at room temperature for 15 min, followed by cell nuclei staining with DAPI for 10 min. Finally, cells were observed by a LSCM (Nikon, C2si, Japan).The cellular uptake efficiency was also analyzed quantitatively by flow cytometry. After 2 h of incubation at 37 °C, cells were washed with cold PBS (pH 7.4) to remove free C6 or C6-loaded NPs, harvested and subsequently resuspended in 0.5 mL PBS for flow cytometrix analysis using FACSCalibur flow cytometry (BD Biosciences, USA) by counting 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells. The autofluorescence of MCF-7 or MCF-7/ADR cells was used as a blank control. All measurements were performed in triplicate.
000 cells. The autofluorescence of MCF-7 or MCF-7/ADR cells was used as a blank control. All measurements were performed in triplicate.
2.11 Cellular accumulation and efflux of Rh123
Rhodamine-123 (Rh123), a typical P-gp substrate, was taken as an index of P-gp activity.9,28 Briefly, MCF-7 and MCF-7/ADR cells were seeded into 6-well black plates at 3 × 105 cells per well and cultured for 24 h. Then, cells were treated with free Rh123 solution, different NPs, and TPGS (5 μg mL−1 Rh123) for 2 h at 37 °C. After that, cells were washed with PBS to remove Rh123, harvested and subsequently resuspended in 0.5 mL PBS. The fluorescence intensity of Rh123 in the cells was analyzed using a FACSCalibur flow cytometry (BD Biosciences, USA). Blank cells were employed as control.For Rh123 efflux assay, MCF-7/ADR cells were seeded into 6-well plates (3 × 105 cells per well) and incubated for 24 h. The cells were treated with 5 μg mL−1 free Rh123 for 1 h at 37 °C. After the pretreatment, cells were exposed to fresh growth medium or blank NPs for another 1 h to allow efflux of the intracellular Rh123. Subsequently, the cells were harvested and measured using the above method.
2.12 Statistical analysis
The data were statistically analysed by using SPSS 17.0 software (Chicago, IL, USA). Statistical analysis was performed as a one-way analysis of variance (ANOVA) and comparisons among groups were performed by independent sample t-tests. The p values <0.05 were considered significantly different. Each experiment was repeated at least three times with a minimum of three samples.3 Results and discussion
3.1 Characterization of CMH copolymer
In this investigation, histidine was conjugated to CMC backbone to produce an amphiphilic copolymer. The schematic of the synthesis of CMH copolymer was illustrated in Fig. S1.† In the analysis of 1H NMR (Fig. 1), the protons of CMC appeared as broad resonances between 2.99 and 5.25 ppm. The proton signals of the acetyl methyl groups were observed at δ = 1.88–2.07 ppm.14,29 The resulting CMH copolymer clearly gave that peaks at 6.81 and 7.62 ppm were ascribed to 4-H (–N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and 2-H (–N
C–) and 2-H (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) imidazole proton of histidine, respectively.18 The signals of imidazole ring confirmed that histidine was successfully attached to CMC-Ac. The number of histidine grafted to 100 saccharide units of CMC-Ac was calculated to be 34.5% based on the ratio of the integral for the signals of imidazole protons to acetyl methyl protons.
CH–) imidazole proton of histidine, respectively.18 The signals of imidazole ring confirmed that histidine was successfully attached to CMC-Ac. The number of histidine grafted to 100 saccharide units of CMC-Ac was calculated to be 34.5% based on the ratio of the integral for the signals of imidazole protons to acetyl methyl protons.
In vitro cytotoxicity and blood-compatibility are two important aspects of a novel drug carrier during the selection process. L929 cells are recommended by many standard institutions as reference cell line for the cytotoxicity testing of polymers.30 Based on results obtained from the MTT assay (Fig. 2A), the polymer cell viability (%) were more than 90% at all concentrations (100–1000 μg mL−1) tested, which implied that the CMH copolymer showed negligible cytotoxic effect towards the normal cells. The degree of hemolysis is shown in Fig. 2B. With the increase of concentration, hemolysis ratio induced by Tween 80 increased dramatically. At concentration of 3 mg mL−1, hemolysis caused by Tween 80 reached 53.9 ± 0.07%, whereas CMH was basically nonhemolytic, exhibiting no more than 4.0% hemolysis. Therefore, it is firmly demonstrated that the well-designed CMH copolymer had better biocompatibility than Tween 80, and could be used in drug delivery system for parenteral administration.
The pH-responsive feature of CMH copolymer was measured in terms of the transmittance at different pH values (Fig. 2C). Above pH 5.0, the transmittance of the CMH solution with light blue opalescence maintained at around 63%. Below pH 5.0, the light transmittance fell sharply and approached 45% at pH 4.0. Accompanied with increased turbidity, we also observed white precipitation, implying aggregation of the polymers. This novel phenomenon was caused by the electrostatic attraction between carboxyl groups and imidazole groups in CMH copolymer. At pH 7.4, the imidazole group was in deprotonated state and hydrophobic,17 while almost all of carboxyl groups on copolymer were in the form of –COO− and hydrophilic.12,31 Therefore, the system stability was promoted by the electrostatic repulsion from carboxylic ions. After exposure to mildly acidic condition, the imidazole group gradually changed from deprotonated state to cationic. The electrostatic attraction between the positively charged imidazole groups and carboxylate anions reduced the net charge of the system, leading to aggregation of the CMH copolymer. The result of zeta potential shed further light on the explanation of pH sensitivity (data not given).
Solubility is considered an important factor that influences the pharmaceutical activity of compounds. It is known that the enhancement of solubility had considerable significance in increasing the bioavailability and efficacy of poor solubility drugs. As presented in Fig. 2D, the solubility of DTX in PBS (pH 7.4) was only 5.41 μg mL−1, whereas DTX could be significantly solubilized by the CMH copolymer. Interestingly, the equilibrium aqueous solubility of DTX in CMH was obviously higher than that in Tween 80 at the same concentration. For example, DTX solubility in 0.1% and 0.3% (w/v) CMH copolymer was found to be 19.51 and 32.13 μg mL−1, respectively, while that in Tween 80 was 10.90 and 27.06 μg mL−1, respectively. The results indicated that the CMH copolymer exhibited better solubilizing capacity for hydrophobic drugs.
3.2 Self-aggregation behaviors of “pure” CMH copolymer and CMH/TPGS mixtures
The CAC is an important parameter affecting self-aggregation behavior and structural stability of nanoparticles in vitro and in vivo. In Fig. S2A,† pyrene excitation spectra of CMH copolymer exhibited a shift from 335 to 338 nm and the total fluorescence intensity increased with the concentration increasing, reflecting that pyrene was transferred from the aqueous polar environment to the less polar microdomain.32 In Fig. S2B,† the fluorescence intensity ratio (I338/I335) against log CMH polymer concentration was plotted, and the CAC was determined to be 0.051 mg mL−1 from the threshold concentration. The findings strongly demonstrated that the amphiphilic CMH copolymer could self-assemble into NPs in aqueous solution. The hydrophobic core of self-aggregates accounted for the significant increase of DTX solubility (Fig. 2D).Three formulations of different CMH to TPGS weight ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 3
1.5, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were prepared, and named as CHT-1, CHT-1.5, and CHT-2, respectively. As shown in Fig. 3A, the CAC values for CHT-1, CHT-1.5, and CHT-2 were 0.0091, 0.0074, and 0.0042 mg mL−1, respectively, which were significantly lower than that of single CMH and TPGS (0.2 mg mL−1). The aromatic ring of TPGS could improve the hydrophobic interactions with amphiphilic copolymers, which further increased the hydrophobic microdomains and solubilized more fluorescent probes, leading to lower CAC values of the hybrid NPs. Such low CAC values could maintain the structural integrity of NPs under highly diluted conditions,6 which highlighted an advantage of the mixed NPs.
2) were prepared, and named as CHT-1, CHT-1.5, and CHT-2, respectively. As shown in Fig. 3A, the CAC values for CHT-1, CHT-1.5, and CHT-2 were 0.0091, 0.0074, and 0.0042 mg mL−1, respectively, which were significantly lower than that of single CMH and TPGS (0.2 mg mL−1). The aromatic ring of TPGS could improve the hydrophobic interactions with amphiphilic copolymers, which further increased the hydrophobic microdomains and solubilized more fluorescent probes, leading to lower CAC values of the hybrid NPs. Such low CAC values could maintain the structural integrity of NPs under highly diluted conditions,6 which highlighted an advantage of the mixed NPs.
3.3 Preparation and characterization of DTX-loaded NPs
The strategy of developing DTX-loaded CMH and CHT NPs was simple and effective. As MeCN evaporated, amphiphilic copolymers self-assembled into NPs in aqueous solution. The hydrophobic DTX was encapsulated into the hydrophobic core of the nanoparticles. The lipophilic alkyl tail of TPGS was incorporated in the polymeric matrix, while hydrophilic PEG component extended outwards into the external aqueous phase. The formation of DTX-loaded CHT NPs might be explained by the hydrophobic interaction between hydrophobic moieties, electrostatic repulsion from carboxylic ions, and steric hindrance resulted by hydrophilic PEG segments.As illustrated in Table 1, it is clearly seen that blank CMH NPs had particle size of about 225 nm with a narrow polydispersity index (PDI < 0.2). Interestingly, a decrease in the particle size after DTX loading was observed for CMH NPs, changing from 225.9 ± 1.74 to 211.0 ± 3.04 nm. We reasoned that encapsulating hydrophobic molecules into NPs enhanced the hydrophobic interaction between the drugs and the hydrophobic core of NPs, leading to more compact and smaller size of the NPs.33–35 In addition, we found that the average particle size of the CMH/TPGS hybrid NPs was much smaller than those of single CMH NPs (p < 0.05). That is because the hybrid NPs formed more tight and compact structures via hydrophobic interaction between CMH copolymer and TPGS.23 The average diameter of the obtained hybrid NPs was found to be less than 200 nm with narrow size distribution. It is known that particle size plays an essential role in determining cellular uptake efficiency and biodistribution. Nanoparticles of size below 200 nm could promote more drug accumulation in tumor issues by the EPR effect.36 Hence, it is suggested that the hybrid NPs fabricated in this study were in the effective range for intended applications. The surface morphology by SEM (Fig. 3B) and TEM image (Fig. 3C) revealed the roughly spherical shape of DTX-loaded CHT-1.5 NPs. The particle size observed from TEM was smaller than that from DLS. Since the NPs measured by DLS were hydrated whereas those measured by TEM were dehydrated.37 Although the particle size observed by SEM also reflected the shrinkage of such NPs, the diameter was larger than that of TEM and close to the DLS analysis. Freeze-drying, which could impact nanoparticle ordering, might account for this phenomenon. The removal of water molecules in lyophilization process allowed nanoparticles to approach each other and then they aggregated via hydrophobic and van der Vaal interaction.39,40 As it turns out, particle size of the reconstituted freeze dried DTX-loaded CHT-1.5 NPs increased to 174.2 ± 4.11 nm.
| Sample | Size (nm) | Polydispersity index (PDI) | Zeta potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| Blank CMH NPs | 225.9 ± 1.74 | 0.17 ± 0.02 | −20.2 ± 1.33 | — | — |
| DTX-loaded CMH NPs | 211.0 ± 3.04 | 0.24 ± 0.02 | −20.9 ± 0.59 | 61.5 ± 3.07 | 4.08 ± 0.03 |
| DTX-loaded CHT-1 NPs | 166.3 ± 2.62 | 0.23 ± 0.01 | −19.4 ± 0.95 | 66.9 ± 2.56 | 3.88 ± 0.15 |
| DTX-loaded CHT-1.5 NPs | 147.8 ± 3.14 | 0.27 ± 0.02 | −17.3 ± 1.21 | 88.3 ± 3.33 | 4.42 ± 0.11 |
| DTX-loaded CHT-2 NPs | 136.6 ± 2.27 | 0.36 ± 0.01 | −16.8 ± 0.17 | 90.1 ± 3.19 | 4.17 ± 0.06 |
It also can be seen from Table 1 that the average zeta potential of all obtained NPs was negative. The reason might be the presence of ionized carboxylic groups in the system, which was helpful to prevent the aggregation of nanoparticles through electrostatic repulsion. Compared with DTX-loaded CMH NPs, the slight decrease in the mean zeta potential of CHT NPs might be ascribed to the masking effect of hydrophilic PEG segment on the nanoparticle surface.41
The CHT-1.5 NPs were chosen as a prototype formulation to ascertain the physical status of DTX in the hybrid NPs. From Fig. 4A, the DSC curve of DTX exhibited an endothermic melting peak at 174.7 °C, while blank CHT-1.5 NPs had only a broad peak at about 200 °C. The endothermic peak of DTX was not observed in the DSC curve of DTX-loaded CHT-1.5 NPs. In the PXRD diagram (Fig. 4B), significant diffraction peaks in DTX which indicated crystalline nature of DTX could be detected at 2θ scattered angles 5.36°, 10.04° and 15.95°. However, no sharp characteristic peaks of pure DTX were identified in DTX-loaded CHT-1.5 NPs, which only showed a wide peak around 12°, just similar to the peak of blank CHT-1.5 NPs. The results demonstrated that DTX was completely imbedded in the hydrophobic microdomain of NPs and might exist as the amorphous or molecular state. The amorphous form might be another important reason for the solubility enhancement of DTX.
3.4 Drug loading and encapsulation capacity
Much research indicated that the major factor influencing the drug loading capacity of polymeric NPs was the compatibility between the drug and core-forming segment.7 The impact of different proportion of TPGS in hybrid NPs on the loading capacity of DTX was determined. From Table 1, it is observed that the EE increased from 61.5 ± 3.07% for 4.08 ± 0.03% DL to 88.32 ± 3.33% for 4.42 ± 0.11% DL when the CMH/TPGS ratio ranging from 3/0 to 3/1.5, which indicated that TPGS played a positive role in drug loading and encapsulation. The additional hydrophobic interaction of DTX with the lipophilic alkyl tail of TPGS improved the compatibility between DTX and the nanoparticle inner core,42 resulting in more hydrophobic drug molecules encapsulated in this hybrid NPs. Nevertheless, the subsequent increase in encapsulation efficiency at higher TPGS level was relatively minor and not significant. The findings led to the assumption that that the weight ratio between CMH and TPGS copolymers at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 could allow for better encapsulation of DTX.
1.5 could allow for better encapsulation of DTX.
3.5 In vitro release assays
The results of in vitro release studies of DTX from “pure” CMH NPs and hybrid NPs in PBS (pH 7.4) were profiled in Fig. 5A. The release percentage of DTX from DTX-loaded CMH NPs was 49.5% within 4 h and nearly 76.4% after 12 h. In contrast, three DTX-loaded CHT NPs exhibited a lower drug release rate than DTX-loaded CMH NPs. At 2 h, the cumulative release of DTX from CHT-1 NPs, CHT-1.5 NPs, and CHT-2 NPs was 17.7%, 9.9%, and 10.0%, respectively. In the following sustained phase, the accumulated amount of drug released from DTX-loaded CHT-1.5 NPs was approximately 75% in 48 h (Fig. 5A). It was obvious that the mixed NPs retained the drug even better than “pure” CMH NPs. This was another advantage of this hybrid nanocarrier when applied to cancer treatment. The pH-dependent drug release behavior of the DTX-loaded NPs was investigated at a physiological pH (7.4) and a lysosomal pH (4.5), as shown in Fig. 5B. A significant difference was noticeable in the DTX release at pH 7.4 and 4.5 (P < 0.05). The DTX-loaded CMH and CHT-1.5 NPs showed a rapid-release phase at pH 4.5 with a cumulative release of 86.5% and 62.7% in 4 h, respectively. Because of the protonation of imidazole groups at low pH values, the CMH-based NPs were disrupted, leading to burst drug release. The faster release of DTX at pH 4.5 suggested that after internalization into tumor cells, the low pH in lysosome might facilitate the release of DTX from polymeric NPs.9,38 | ||
| Fig. 5 (A) In vitro release of DTX from CMH and CHT NPs at pH 7.4. (B) In vitro release of DTX from CMH and CHT-1.5 NPs in PBS (pH 7.4) and acetate buffer (pH 4.5). Data are mean ± SD (n = 3). | ||
DTX being highly hydrophobic was sequestered inside the core of the engineered NPs. The release profile of DTX from CHT-1.5 NPs in PBS (pH 7.4) were fitted with five different model equations, including first kinetics, Higuchi, Baker–Lonsdale, Ritger–Peppas, and Hixcon–Crowell equations. The calculated correlation coefficient (r) was 0.9673, 0.9788, 0.9790, 0.9936, and 0.9318, respectively. The kinetics of DTX conformed well to Higuchi, Baker–Lonsdale, and Ritger–Peppas models, the mechanism of which mainly depended on the diffusion.
3.6 Cell viability studies
The CHT-1.5 NPs with better pharmaceutical properties were selected for further study. To determine whether the well-engineered mixed NPs could improve the treatment of MDR, DTX-loaded CHT-1.5 NPs was tested against MCF-7 and P-gp over-expressing MCF-7/ADR cells by the MTT assay. As shown in Fig. 6, in the concentration ranges used in this study, the cytotoxicity of CMH copolymer towards the cell lines was negligible. The blank CHT-1.5 NPs showed certain inhibition of both MCF-7 and MCF-7/ADR cell growth with an increase in polymer concentration, suggesting a synergistic effect between this nanocarrier and anticancer drugs. It has been well verified that TPGS exhibits selective cytotoxicity for cancer cells, which could lead to apoptosis in breast cancer cells with less or no toxicity in most normal cells.21As shown in Fig. 6A and B, the cytotoxicity of DTX in the drug-resistant MCF-7/ADR cells was limited compared with that in drug-sensitive MCF-7 cells. This result was associated with the large amounts of P-gp expressed by MCF-7/ADR cells, which could extrude the substrates out of the resistant cancer cells.43,44 However, DTX-loaded CHT-1.5 NPs were more effective than DTX-loaded CMH NPs and free DTX in killing tumor cells, especially in drug-resistant MCF-7/ADR cells. The RI and RF were calculated to quantitatively assess the reversal effect on MDR by DTX-loaded CHT-1.5 NPs. As shown in Table 2, the RI value of free DTX was 35.49, suggesting the good cross resistance of MCF-7/ADR cells to DTX. Interestingly, the RI value of DTX-loaded CMH and CHT-1.5 NPs decreased to 30.22 and 11.21 respectively. The partial reversal effect revealed by CMH NPs might be attributed to the pH triggered faster drug release (Fig. 4) and the endocytosis of polymeric NPs.38 It is reported that polymeric NPs can inherently circumvent P-gp mediated efflux through endocytosis, partially reducing the multidrug resistance.45,46 More importantly, DTX-loaded CHT-1.5 NPs showed much higher RF values than DTX-loaded CMH NPs, demonstrating the best MDR reversal effect on MDR cells was related to the presence of TPGS, which could block the function of P-gp by ATPase inhibition to achieve the MDR reversal effect.41,47,48
| Sample | IC50 | RI | RF | |
|---|---|---|---|---|
| MCF-7 | MCF-7/ADR | |||
| Free DTX | 0.69 ± 0.04 | 24.49 ± 1.16 | 35.49 | 1.00 |
| DTX-loaded CMH NPs | 0.72 ± 0.09 | 21.76 ± 1.17 | 30.22 | 1.13 |
| DTX-loaded CHT-1.5 NPs | 0.43 ± 0.07 | 4.82 ± 0.07 | 11.21 | 5.08 |
3.7 In vitro cellular uptake assays
In this study, C6 as a fluorescent probe was used to represent DTX in the NPs to visualize and analyze cellular uptake of the NPs. Cellular uptake efficiency of C6-loaded CHT-1.5 NPs was qualitatively evaluated by CLSM, as listed in Fig. 7. Free C6 and C6-labeled NPs were detected by the FITC channel. The nuclei were stained with DAPI and detected by the DAPI channel. All images showed the merged channels of FITC and DAPI channels. The outlines of the cells were clearly showed in the bright field. As shown in Fig. 7, C6-loaded NPs (green) were closely located around the nuclei (blue, stained by DAPI), indicating the fluorescent NPs had been internalized into the cells. Compared with MCF-7 cells, the relatively lower fluorescence intensity was found in MCF-7/ADR cells, resulting from the P-gp mediated drug efflux. As expected, the C6-loaded CHT-1.5 NPs exhibited much stronger fluorescence intensity compared with C6-loaded CMH NPs and free C6 in MCF-7/ADR cells. The finding suggested that CHT-1.5 NPs could significantly enhance drug intracellular accumulation in MCF-7/ADR cells and overcome MDR.Flow cytometry was employed to further quantify the uptake levels of the NPs. Cells without C6 treatment were used as a control that showed the autofluorescence. As can be seen in Fig. 8A and B, the cellular uptake of free C6 was severely limited in drug-resistant MCF-7/ADR cells compared with that in MCF-7 cells, which was in accordance with the CLSM images. The specific geometric mean fluorescent intensities of free C6 group in MCF-7 cells were 2.79-fold greater than that in MCF-7/ADR cells. Interestingly, the mean fluorescent intensities of C6-loaded CMH and CHT-1.5 NPs in MCF-7 cells were 1.77- and1.17-fold higher than that in MCF-7/ADR cells respectively, demonstrating that the polymeric NPs could enhance cellular uptake and partially overcome drug resistance. Free C6 solution may transport across the cell membrane through passive diffusion, a pathway that was affected in drug-resistant cells by P-gp over-expression.49,50 In contrast, drug entrapped in polymeric NPs probably enters cancer cells through endocytosis, a pathway that can inherently circumvent P-gp mediated efflux, resulting in increased intracellular drug accumulation in the cytosol and better cytotoxicity efficacy in drug-resistant cells.45 The above results were in good agreement with the MTT assay. In addition, CHT-1.5 NPs displayed higher C6 uptake efficiency than CMH NPs in MCF-7 cells, which could be ascribed to its smaller particle size (Table 1). It has been proved that the mechanism of cellular uptake of polymeric NPs is size dependent.51 Notably, the CHT-1.5 NPs resulted in 1.89-fold higher uptake level of C6 than free C6 group (P < 0.05) and 1.79-fold higher than CMH NPs (p < 0.05) in MCF-7/ADR cells. The high amount of cellular uptake of CHT-1.5 NPs might be caused by synergistic effects of the bypassing of the P-gp efflux pump by endocytosis, smaller particle size, and inhibition of P-gp activity.
3.8 Effects of CHT-1.5 NPs on P-gp activities in MCF-7/ADR cells
To test the effect of CHT-1.5 NPs on the P-gp activity, the cellular accumulation and efflux of Rh123, a substrate of P-gp, were measured using flow cytometer respectively. Cell accumulation of Rh123 with TPGS treatment (P-gp efflux inhibitor) was used as positive control. As shown in Fig. 8C, the results indicated that the MCF-7/ADR cells showed similar Rh123 accumulation after incubation with free Rh123 and CMH copolymer (P > 0.05), indicating that CMH NPs could not improve drug retention. Hence we assumed that the CMH copolymer did not affect the inhibition of P-gp activity. In contrast, the P-gp overexpressing MCF-7/ADR cells treated with either CHT-1.5 NPs or TPGS showed significantly enhanced Rh123 cellular accumulation than those incubated with CMH and free Rh123 (P < 0.05), suggesting that TPGS in the hybrid NPs played main role in inhibition of P-gp activity. In addition, similar results were observed in Rh123 efflux test (Fig. 8D). The mean fluorescence intensity of Rh123 was not significantly changed in cells treated with CMH copolymer (P > 0.05), but obviously improved after the treatment of CHT-1.5 NPs or TPGS (P < 0.05). This further demonstrated the ability of the TPGS copolymer in CHT-1.5 NPs to inhibit P-gp mediated drug efflux. The possible mechanism of TPGS on the inhibition of ATPase activity was related to reduction of mitochondrial membrane potential and ATP level in the cells, but without inhibition of P-gp expression on multidrug-resistant cells.9,52,534 Conclusion
In this research, the well-designed multifunctional NPs integrated the superiorities of “smart” CMH and TPGS copolymers for effective delivery of DTX and overcoming MDR. By the synergistic effects of the CMH and TPGS, CHT-1.5 NPs achieved lower CMC values, smaller particle size, higher drug encapsulation, and pH-sensitive release profile. Furthermore, the DTX-loaded CHT-1.5 NPs achieved significantly higher level of cytotoxicity against MCF-7 and MCF-7/ADR cells than free DTX and DTX-loaded CMH NPs. The high amount of cellular uptake of CHT-1.5 NPs in MCF-7/ADR cells was caused by the combination of the evading MDR efflux pumps through endocytosis, smaller nanoparticle size, and significant inhibition of the P-gp mediated efflux system. Therefore, the CHT-1.5 NPs can act as a potential delivery carrier to enhance therapeutic effect and overcome MDR in P-gp overexpressing tumor cells.Notes and references
- M. T. Huizing, V. H. Misser, R. C. Pieters, W. W. ten Bokkel Huinink, C. H. Veenhof, J. B. Vermorken, H. M. Pinedo and J. H. Beijnen, Cancer Invest., 1995, 13, 381–404 CrossRef CAS.
- B. Nuijen, M. Bouma, J. H. Schellens and J. H. Beijnen, Invest. New Drugs, 2001, 19, 143–153 CrossRef CAS.
- M. M. Gottesman, T. Fojo and S. E. Bates, Nat. Rev. Cancer, 2002, 2, 48–58 CrossRef CAS PubMed.
- Y. Kimura, S. Morita, M. Matsuo and K. Ueda, Cancer Sci., 2007, 98, 1303–1310 CrossRef CAS PubMed.
- A. Roy, M. Murakami, M. J. Ernsting, B. Hoang, E. Undzys and S. D. Li, Mol. Pharm., 2014, 11, 2592–2599 CrossRef CAS PubMed.
- L. P. Qiu, Z. Li, M. X. Qiao, M. M. Long, M. Y. Wang, X. J. Zhang, C. M. Tian and D. W. Chen, Acta Biomater., 2014, 10, 2024–2035 CrossRef CAS PubMed.
- C. F. Mu, P. Balakrishnan, F. D. Cui, Y. M. Yin, Y. B. Lee, H. G. Choi, C. S. Yong, S. J. Chung, C. K. Shim and D. D. Kim, Biomaterials, 2010, 31, 2371–2379 CrossRef CAS PubMed.
- L. Mei, Y. Q. Zhang, Y. Zheng, G. Tian, C. X. Song, D. Y. Yang, H. L. Chen, H. F. Sun, Y. Tian, K. X. Liu, Z. Li and L. Q. Huang, Nanoscale Res. Lett., 2009, 4, 1530–1539 CrossRef CAS PubMed.
- L. Qiu, M. Qiao, Q. Chen, C. Tian, M. Long, M. Wang, Z. Li, W. Hu, G. Li, L. Cheng, L. Cheng, H. Hu, X. Zhao and D. Chen, Biomaterials, 2014, 35, 9877–9887 CrossRef CAS PubMed.
- L. Mu, T. A. Elbayoumi and V. P. Torchilin, Int. J. Pharm., 2005, 306, 142–149 CrossRef CAS PubMed.
- L. Mu and S. S. Feng, Pharm. Res., 2003, 20, 1864–1872 CrossRef CAS.
- L. Q. Yang, J. L. Kuang, J. Wang, Z. Q. Li and L. M. Zhang, Macromol. Biosci., 2008, 8, 279–286 CrossRef CAS PubMed.
- K. Zhu, T. Ye, J. Liu, Z. Peng, S. Xu, J. Lei, H. Deng and B. Li, Int. J. Pharm., 2013, 441, 721–727 CrossRef CAS PubMed.
- M. J. Ernsting, W. L. Tang, N. MacCallum and S. D. Li, Bioconjugate Chem., 2011, 22, 2474–2486 CrossRef CAS PubMed.
- M. J. Ernsting, W. L. Tang, N. W. MacCallum and S. D. Li, Biomaterials, 2012, 33, 1445–1454 CrossRef CAS PubMed.
- M. J. Ernsting, M. Murakami, E. Undzys, A. Aman, B. Press and S. D. Li, J. Controlled Release, 2012, 162, 575–581 CrossRef CAS PubMed.
- J. L. Wu, C. G. Liu, X. L. Wang and Z. H. Huang, J. Mater. Sci.: Mater. Med., 2012, 23, 1921–1929 CrossRef CAS PubMed.
- J. J. Gu, X. Wang, X. Y. Jiang, Y. Z. Chen, L. C. Chen, X. L. Fang and X. Y. Sha, Biomaterials, 2012, 33, 644–658 CrossRef CAS PubMed.
- Z. P. Zhang, S. W. Tan and S. S. Feng, Biomaterials, 2012, 33, 4889–4906 CrossRef CAS PubMed.
- Y. Y. Guo, J. Luo, S. W. Tan, B. O. Otieno and Z. P. Zhang, Eur. J. Pharm. Sci., 2013, 49, 175–186 CrossRef CAS PubMed.
- C. M. Neophytou, C. Constantinou, P. Papageorgis and A. I. Constantinou, Biochem. Pharmacol., 2014, 89, 31–42 CrossRef CAS PubMed.
- Y. Mi, Y. T. Liu and S. S. Feng, Biomaterials, 2011, 32, 4058–4066 CrossRef CAS PubMed.
- D. Pooja, H. Kulhari, M. K. Singh, S. Mukherjee, S. S. Rachamalla and R. Sistla, Colloids Surf., B, 2014, 121, 461–468 CrossRef CAS PubMed.
- K. K. Gill, A. Kaddoumi and S. Nazzal, Eur. J. Pharm. Sci., 2012, 46, 64–71 CrossRef CAS PubMed.
- X. Li, X. Tian, J. Zhang, X. Zhao, X. H. Chen, Y. H. Jiang, D. K. Wang and W. S. Pan, Int. J. Nanomed., 2011, 6, 1167–1184 CAS.
- H. Lee, K. Lee and T. G. Park, Bioconjugate Chem., 2008, 19, 1319–1325 CrossRef CAS PubMed.
- M. S. Muthu, S. A. Kulkarni, J. Q. Xiong and S. S. Feng, Int. J. Pharm., 2011, 421, 332–340 CrossRef CAS PubMed.
- Y. Wang, L. M. Dou, H. J. He, Y. Zhang and Q. Shen, Mol. Pharmaceutics, 2014, 11, 885–894 CrossRef CAS PubMed.
- H. T. Pang, X. G. Chen, H. J. Park, D. S. Cha and J. F. Kennedy, Carbohydr. Polym., 2007, 69, 419–425 CrossRef CAS PubMed.
- D. Fischer, Y. X. Li, B. Ahlemeyer, J. Krieglstein and T. Kissel, Biomaterials, 2003, 24, 1121–1131 CrossRef CAS.
- E. Arinaitwe and M. Pawlik, Carbohydr. Polym., 2014, 99, 423–431 CrossRef CAS PubMed.
- R. Tang, W. Ji, D. Panus, R. N. Palumbo and C. Wang, J. Controlled Release, 2011, 151, 18–27 CrossRef CAS PubMed.
- J. Li, M. Huo, J. Wang, J. Zhou, J. M. Mohammad, Y. Zhang, Q. Zhu, A. Y. Waddad and Q. Zhang, Biomaterials, 2012, 33, 2310–2320 CrossRef CAS PubMed.
- M. J. Shieh, C. Y. Hsu, L. Y. Huang, H. Y. Chen, F. H. Huang and P. S. Lai, J. Controlled Release, 2011, 152, 418–425 CrossRef CAS PubMed.
- D. de Melo-Diogo, V. M. Gaspar, E. C. Costa, A. F. Moreira, D. Oppolzer, E. Gallardo and I. J. Correia, Eur. J. Pharm. Biopharm., 2014, 88, 718–729 CrossRef CAS PubMed.
- J. B. Huang, H. Zhang, Y. Yu, Y. Chen, D. Wang, G. Q. Zhang, G. C. Zhou, J. J. Liu, Z. G. Sun, D. X. Sun, Y. Lu and Y. Q. Zhong, Biomaterials, 2014, 35, 550–566 CrossRef CAS PubMed.
- F. Qiu, J. Feng, D. Q. Wu, X. Z. Zhang and R. X. Zhuo, Eur. Polym. J., 2009, 45, 1024–1031 CrossRef CAS PubMed.
- Z. Li, L. P. Qiu, Q. Chen, T. N. Hao, M. X. Qiao, H. X. Zhao, J. Zhang, H. Y. Hu, X. L. Zhao, D. W. Chen and L. Mei, Acta Biomater., 2015, 11, 137–150 CrossRef CAS PubMed.
- Z. Teng, Y. C. Luo and Q. Wang, Food Chem., 2013, 141, 524–532 CrossRef CAS PubMed.
- Y. Y. Guo, M. Chu, S. W. Tan, S. Zhao, H. X. Liu, B. O. Otieno, X. L. Yang, C. R. Xu and Z. P. Zhang, Mol. Pharmaceutics, 2014, 11, 59–70 CrossRef CAS PubMed.
- S. Zhao, S. W. Tan, Y. Y. Guo, J. Huang, M. Chu, H. D. Liu and Z. P. Zhang, Biomacromolecules, 2013, 14, 2636–2646 CrossRef CAS PubMed.
- S. L. Huang, X. H. Yu, L. L. Yang, F. L. Song, G. Chen, Z. F. Lv, T. Li, D. Chen, W. H. Zhu, A. A. Yu, Y. M. Zhang and F. Yang, Eur. J. Pharm. Sci., 2014, 63, 187–198 CrossRef CAS PubMed.
- H. Wang, T. Vo, A. Hajar, S. Li, X. M. Chen, A. M. Parissenti, D. N. Brindley and Z. X. Wang, BMC Cancer, 2014, 14, 1–15 CrossRef PubMed.
- P. Y. Li, P. S. Lai, W. C. Hung and W. J. Syu, Biomacromolecules, 2010, 11, 2576–2582 CrossRef CAS PubMed.
- H. L. Wong, R. Bendayan, A. M. Rauth, H. Y. Xue, K. Babakhanian and X. Y. Wu, J. Pharmacol. Exp. Ther., 2006, 317, 1372–1381 CrossRef CAS PubMed.
- S. P. Wang, J. G. Qiu, Z. Shi, Y. T. Wang and M. W. Chen, Biotechnol. Adv., 2015, 33, 224–241 CrossRef CAS PubMed.
- L. P. Qiu, M. X. Qiao, Q. Chen, C. M. Tian, M. M. Long, M. Y. Wang, Z. Li, W. Hu, G. Li, L. Cheng, L. F. Cheng, H. Y. Hu, X. L. Zhao and D. W. Chen, Biomaterials, 2014, 35, 9877–9887 CrossRef CAS PubMed.
- E. M. Collnot, C. Baldes, M. F. Wempe, R. Kappl, J. Huttermann, J. A. Hyatt, K. J. Edgar, U. F. Schaefer and C. M. Lehr, Mol. Pharm., 2007, 4, 465–474 CrossRef CAS PubMed.
- A. Mahmud and A. Lavasanifar, Colloids Surf., B, 2005, 45, 82–89 CrossRef CAS PubMed.
- P. R. Wielinga, H. V. Westerhoff and J. Lankelma, Eur. J. Biochem., 2000, 267, 649–657 CrossRef CAS.
- X. Zhang, Y. Yang, X. Liang, X. Zeng, Z. Liu, W. Tao, X. Xiao, H. Chen, L. Huang and L. Mei, Theranostics, 2014, 4, 1085–1095 CrossRef PubMed.
- P. Yu, H. Yu, C. Guo, Z. Cui, X. Chen, Q. Yin, P. Zhang, X. Yang, H. Cui and Y. Li, Acta Biomater., 2015, 14, 115–124 CrossRef CAS PubMed.
- H. H. Bu, X. Y. He, Z. W. Zhang, Q. Yin, H. J. Yu and Y. P. Li, Int. J. Pharm., 2014, 471, 206–213 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra05586e |
| This journal is © The Royal Society of Chemistry 2015 |