13C pathway analysis of biofilm metabolism of Shewanella oneidensis MR-1
Weihua Guo†
a,
Shuai Luo†b,
Zhen He*b and
Xueyang Feng*a
aDepartment of Biological Systems Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA. E-mail: xueyang@vt.edu; Tel: +1-540-231-2974
bDepartment of Civil and Environmental Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA. E-mail: zhenhe@vt.edu; Tel: +1-540-231-1346
First published on 24th April 2015
Abstract
Biofilm metabolism of Shewanella was analyzed via 13C tracing experiments for the first time. The activity of C1 metabolism in the biofilm cells was found to be interestingly higher than that in planktonic cells, which could be related to utilizing C1 metabolites as electron donors when growing Shewanella in biofilms.
The biofilms formed by Shewanella oneidensis MR-1 have been extensively studied1–5 and found to play pivotal roles in bioremediation of heavy metals6–10 and electric power generation.11,12 Despite recent discoveries of Shewanella metabolism under aerobic and anaerobic conditions using various analytical approaches,13–16 few studies have been accomplished to investigate the metabolic pathway usage in Shewanella biofilms. In this study, we applied the 13C pathway analysis, a reliable and well-developed technology,17 to analyze the carbon metabolism of S. oneidensis cells derived from biofilm and planktonic growth, respectively. Through the comparison of isotopomer labeling patterns of key proteinogenic amino acids, we found that the C1 metabolism was much more active when growing S. oneidensis in biofilms compared to planktonic cells, which could be related to the utilization of C1 metabolites as electron donor by S. oneidensis when growing in biofilms. To our best knowledge, this is the first time that the biofilm metabolism of S. oneidensis was rigorously determined by isotopomer analysis.
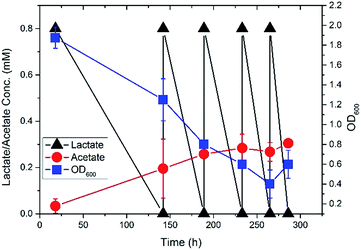
S. oneidensis MR-1 was initially grown in shake flasks with minimal medium containing 3.7 mM [3-13C] sodium L-lactate (Sigma-Aldrich) for two days at 100 rpm, 30 °C. It was then transferred to completely fill up a 140 mL sealed bottle reactor and cultivated under oxygen-limited condition (stirrer at the bottom) at 30 °C. To grow S. oneidensis MR-1 in a biofilm, a carbon cloth (2.5 cm × 4.5 cm, Zoltek, Panex® 30 Fabric, PW06) was submerged in the medium of the sealed bottle reactor, with titanium mesh (McMaster) to support and titanium wire (Sigma-Aldrich) to bind the cloth tightly on mesh. Duplicate reactors were processed (n = 2). When lactate was depleted (<0.01 mM), the minimal medium was refreshed by removing 10 mL medium from the sealed bottle reactor and injecting 10 mL fresh filter (0.22 μm pore size) sterilized medium. The final concentration of [3-13C] sodium L-lactate was maintained at ∼0.8 mM. The biomass of planktonic cells was monitored by OD600 using a plate reader (BioTek). The concentrations of lactate and acetate in the sealed bottle reactor were measured by high-performance liquid chromatography (HPLC, Shimadzu), following the method that has been previously developed18 (Fig. 1). It was found that lactate was consumed to produce acetate as the sole fermentation byproduct when growing Shewanella in the sealed bottle bioreactor. The declining OD600 of the planktonic cells in the reactor indicated that more cells would be grown in the biofilms with the replenishment of the medium.
After five refreshment of the medium, both the planktonic cells and the biofilm cells were collected in duplicate from the liquid culture and the carbon cloth, respectively, followed by isotopomer analysis of proteiogenic amino acids using a previously developed protocol.19–21 In general, the biomass was hydrolysed using 6 M HCl (24 h at 100 °C). The amino acids were derivatized in 20 μL of tetrahydrofuran and 20 μL of N-(tert-butyldimethylsilyl)-N-methyl-trifluoroacetamide (Sigma-Aldrich). A gas chromatograph (GC2010, Shimadzu) equipped with a SH-Rxi-5Sil column (Shimadzu) and a mass spectrometer (QP2010, Shimadzu) was used for analyzing the labeling profiles of metabolites. Three types of charged fragments were detected by GC-MS for Ala, Gly, Ser, Asp and Glu: the [M-57]+ group (containing unfragmented amino acids); and the [M-159]+ or [M-85]+ group (containing amino acids that had lost an α-carboxyl group). For each type of fragments, the labeling patterns were represented by M0, M1, M2, etc., which were fractions of non-labeled, singly labeled, and doubly labeled amino acids. The effects of natural isotopes on isotopomer labeling patterns were corrected by previously reported algorithms.22
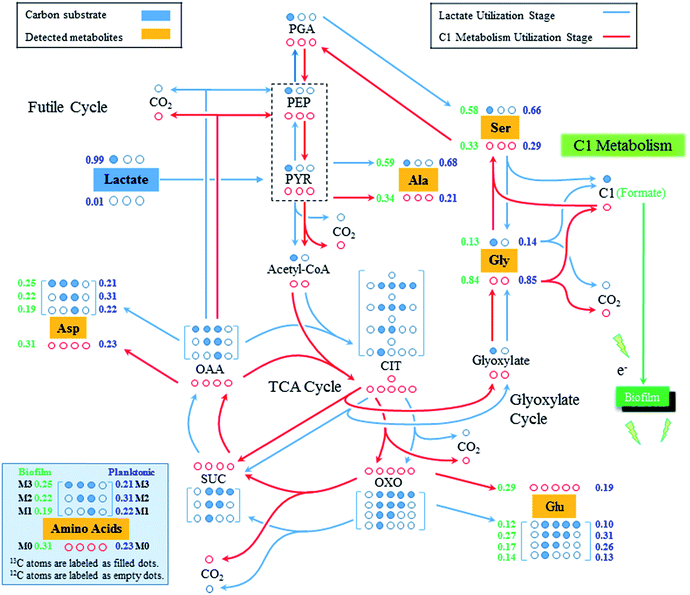
By feeding [3-13C] lactate as the sole carbon source to S. oneidensis under an oxygen-limited culture condition, most alanine molecules were expected to be singly labeled because pyruvate, the direct precursor of alanine, was believed to be mainly synthesized from [3-13C] lactate (Fig. 2 and Table 1). To our surprise, 34% alanine molecules were found to be non-labeled in the biofilm cells. As a result of the non-labeled pyruvate produced, we also found a significant amount of non-labeled aspartate and glutamate in the biofilm cells, which were synthesized from the futile cycle and the TCA cycle, respectively, by using the non-labeled pyruvate as the precursor. However, it remains unknown that how the non-labeled pyruvate was synthesized during Shewanella growth in biofilms.
| Biofilm samples | Planktonic samples | |||
|---|---|---|---|---|
| M-57 | M-159/85 | M-57 | M-159/85 | |
| a The error in mass distribution from duplicates was <5%. | ||||
| Ala | ||||
| M0 | 0.34 | 0.35 | 0.21 | 0.22 |
| M1 | 0.59 | 0.58 | 0.68 | 0.70 |
| M2 | 0.05 | 0.07 | 0.09 | 0.08 |
| M3 | 0.01 | 0.02 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Gly | ||||
| M0 | 0.84 | 0.87 | 0.85 | 0.88 |
| M1 | 0.13 | 0.13 | 0.14 | 0.12 |
| M2 | 0.03 | 0.01 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ser | ||||
| M0 | 0.33 | 0.39 | 0.29 | 0.29 |
| M1 | 0.58 | 0.56 | 0.66 | 0.66 |
| M2 | 0.08 | 0.06 | 0.04 | 0.05 |
| M3 | 0.01 | 0.01 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Asp | ||||
| M0 | 0.31 | 0.33 | 0.23 | 0.23 |
| M1 | 0.19 | 0.25 | 0.22 | 0.31 |
| M2 | 0.22 | 0.28 | 0.31 | 0.31 |
| M3 | 0.25 | 0.14 | 0.21 | 0.15 |
| M4 | 0.02 | 0.02 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Glu | ||||
| M0 | 0.28 | 0.29 | 0.19 | 0.20 |
| M1 | 0.14 | 0.16 | 0.13 | 0.16 |
| M2 | 0.17 | 0.26 | 0.26 | 0.33 |
| M3 | 0.27 | 0.27 | 0.31 | 0.30 |
| M4 | 0.12 | 0.03 | 0.10 | 0.02 |
| M5 | 0.02 | 0.00 | ||
To our best knowledge, there is only one pathway, C1 metabolism, which could lead to the synthesis of non-labeled pyruvate when growing with [3-13C] lactate.3,23–25 Generally, pyruvate could be converted to 3-phosphoglycerate (PGA) through the gluconeogenesis, which is used to produce serine, and further converted into glycine and a C1 molecule (e.g., formate) via C1 metabolism. During the glycine synthesis from serine, the labeled carbon originated from the [3-13C] lactate would get lost as C1 metabolites while glycine became mostly non-labeled, as found in this study (Fig. 2). Glycine could be continuously divided into CO2 and C1 molecule, both of which were non-labeled in this study. Considering the high reversibility of the C1 metabolism, the non-labeled C1 metabolites could be incorporated with non-labeled glycine to synthesize non-labeled serine (i.e., reversed C1 metabolism), which would then be used to synthesize the non-labeled pyruvate via serine dehydratase (SDH, EC 4.3.1.17).26,27 This is possible in the present study because we grew Shewanella in biofilms with very low concentration of [3-13C] lactate (∼0.8 mM), which was rapidly consumed for cell growth within 5–10 hours but also produced non-labeled C1 metabolites via the C1 metabolic pathway. Once lactate utilization stage was finished, Shewanella growing in biofilms would use the generated C1 metabolites as the secondary carbon source to continue the production of building blocks for cell growth. As a result of the combined effects of lactate utilization and C1 metabolites utilization, the proteinogenic amino acids, such as alanine, aspartate and glutamate, demonstrated a high percentage of non-labeled molecules (34%, 31%, and 28%, respectively). It is also worth noting that although most of detected glycine (84%) is non-labeled, there was 13% single-labeled glycine detected, which could be synthesized from glyoxylate shunt of TCA cycle as that has been discovered in previous studies.3,23,25
Compared to the isotopomer labeling patterns of the biofilm cells, the non-labeled proteinogenic amino acids (e.g., Ala, Glu, and Asp) were also detected in the planktonic cells of S. oneidensis MR-1, which could come from two sources. First, the C1 metabolism could be active in the planktonic cells and generate non-labeled amino acids. Secondly, considering the cohesiveness of the biofilm, some cells could be washed out from the biofilm and become the planktonic cells,28,29 which brought non-labeled amino acids as detected in the planktonic cells. Indeed, at the end of the fifth medium replenishment, we did observe an increased OD600 of the planktonic cells (Fig. 1), which could be attributed to the washout of the biofilm cells. The washout was not observed in early stages since the cells attached on biofilms were not saturated, while in the late stages, the biofilm could no longer attach all the cells and the extra cells were washed out. We also found that the percentages of non-labeled proteinogenic amino acids in planktonic cells were much smaller compared to that in biofilm cells. For example, only 23% of alanine detected in the planktonic cells was non-labeled while 34% alanine was found to be non-labeled in biofilm cells. This is possible since the substrate (i.e., [3-13C] lactate in this study) was more difficult to diffuse into the biofilms, more lactate would be used by the planktonic cells, which makes the lactate utilization metabolism more dominant in the planktonic cells and leads to smaller percentage of non-labeled pyruvate synthesized from C1 metabolism of planktonic cells.
The results of this study have important implications to use S. oneidensis MR-1 for energy production. For example, it has been well known that by forming the biofilms, S. oneidensis MR-1 could generate electricity from organic carbon substrates.12,30 The discovery from this study indicated that the C1 metabolite, especially formate, could serve as the electron donor and generate electricity during the C1 metabolism.24 Considering the direct connection of biofilm cells and carbon cloth, the electron transfer of biofilm cells should be more active compared to planktonic cells, which could lead to more active C1 metabolism in the biofilm cells. The effects on C1 metabolites (e.g., formate) on bioelectricity generation and the exact pathway of carbon flux during bioelectricity generation are currently being investigated and expected to be reported in future. In summary, by applying 13C pathway analysis to investigate microbial metabolism of S. oneidensis MR-1 growing in biofilms, we discovered that the activity of C1 metabolism was interestingly higher than that in planktonic cells, which could be related to the utilization of C1 metabolites as electron donor when growing Shewanella in biofilms.
Notes and references
- A. S. Beliaev, D. K. Thompson, T. Khare, H. Lim, C. C. Brandt, G. Li, A. E. Murray, J. F. Heidelberg, C. S. Giometti and J. Yates III, OMICS, 2002, 6, 39–60 CrossRef CAS.
- J. F. Heidelberg, I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson and C. M. Fraser, Nat. Biotechnol., 2002, 20, 1118–1123 CrossRef CAS PubMed.
- Y. J. Tang, J. S. Hwang, D. E. Wemmer and J. D. Keasling, Appl. Environ. Microbiol., 2007, 73, 718–729 CrossRef CAS PubMed.
- J. S. McLean, P. D. Majors, C. L. Reardon, C. L. Bilskis, S. B. Reed, M. F. Romine and J. K. Fredrickson, J. Microbiol. Methods, 2008, 74, 47–56 CrossRef CAS PubMed.
- G. E. Pinchuk, E. A. Hill, O. V. Geydebrekht, J. De Ingeniis, X. Zhang, A. Osterman, J. H. Scott, S. B. Reed, M. F. Romine, A. E. Konopka, A. S. Beliaev, J. K. Fredrickson and J. L. Reed, PLoS Comput. Biol., 2010, 6, e1000822 Search PubMed.
- J. M. Tiedje, Nat. Biotechnol., 2002, 20, 1093–1094 CrossRef CAS PubMed.
- T. DiChristina, D. Bates, J. Burns, J. Dale and A. Payne, in Past and Present Water Column Anoxia, ed. L. N. Neretin, Springer, Netherlands, 2006, vol. 64, ch. 17, pp. 443–469 Search PubMed.
- H. H. Hau and J. A. Gralnick, Annu. Rev. Microbiol., 2007, 61, 237–258 CrossRef CAS PubMed.
- J. K. Fredrickson, M. F. Romine, A. S. Beliaev, J. M. Auchtung, M. E. Driscoll, T. S. Gardner, K. H. Nealson, A. L. Osterman, G. Pinchuk, J. L. Reed, D. A. Rodionov, J. L. M. Rodrigues, D. A. Saffarini, M. H. Serres, A. M. Spormann, I. B. Zhulin and J. M. Tiedje, Nat. Rev. Microbiol., 2008, 6, 592–603 CrossRef CAS PubMed.
- H. H. Hau, A. Gilbert, D. Coursolle and J. A. Gralnick, Appl. Environ. Microbiol., 2008, 74, 6880–6886 CrossRef CAS PubMed.
- G. J. Newton, S. Mori, R. Nakamura, K. Hashimoto and K. Watanabe, Appl. Environ. Microbiol., 2009, 75, 7674–7681 CrossRef CAS PubMed.
- V. J. Watson and B. E. Logan, Biotechnol. Bioeng., 2010, 105, 489–498 CrossRef CAS PubMed.
- R. Renslow, J. Babauta, A. Kuprat, J. Schenk, C. Ivory, J. Fredrickson and H. Beyenal, Phys. Chem. Chem. Phys., 2013, 15, 19262–19283 RSC.
- Y. Ding, N. Peng, Y. Du, L. Ji and B. Cao, Appl. Environ. Microbiol., 2014, 80, 1498–1506 CrossRef CAS PubMed.
- C. Grobbler, B. Virdis, A. Nouwens, F. Harnisch, K. Rabaey and P. L. Bond, Syst. Appl. Microbiol., 2014, 38, 135–139 CrossRef PubMed.
- Y. Zhang, C. K. Ng, Y. Cohen and B. Cao, Mol. BioSyst., 2014, 10, 1035–1042 RSC.
- Y. J. Tang, H. G. Martin, S. Myers, S. Rodriguez, E. E. Baidoo and J. D. Keasling, Mass Spectrom. Rev., 2009, 28, 362–375 CrossRef CAS PubMed.
- G. J. Newton, S. Mori, R. Nakamura, K. Hashimoto and K. Watanabe, Appl. Environ. Microbiol., 2009, 75, 7674–7681 CrossRef CAS PubMed.
- X. Feng and H. Zhao, Microb. Cell Fact., 2013, 12, 114 CrossRef PubMed.
- X. Feng, K.-H. Tang, R. E. Blankenship and Y. J. Tang, J. Biol. Chem., 2010, 285, 39544–39550 CrossRef CAS PubMed.
- X. Feng, H. Mouttaki, L. Lin, R. Huang, B. Wu, C. L. Hemme, Z. He, B. Zhang, L. M. Hicks and J. Xu, Appl. Environ. Microbiol., 2009, 75, 5001–5008 CrossRef CAS PubMed.
- S. A. Wahl, M. Dauner and W. Wiechert, Biotechnol. Bioeng., 2004, 85, 259–268 CrossRef CAS PubMed.
- J. H. Scott and K. H. Nealson, J. Bacteriol., 1994, 176, 3408–3411 CAS.
- M. H. Serres and M. Riley, J. Bacteriol., 2006, 188, 4601–4609 CrossRef CAS PubMed.
- Y. J. Tang, A. L. Meadows, J. Kirby and J. D. Keasling, J. Bacteriol., 2007, 189, 894–901 CrossRef CAS PubMed.
- D. Simon, J. Hoshino and H. Kröger, Biochim. Biophys. Acta, Enzymol., 1973, 321, 361–368 CrossRef CAS.
- R. Grabowski, A. E. Hofmeister and W. Buckel, Trends Biochem. Sci., 1993, 18, 297–300 CrossRef CAS.
- M. Ghannoum and G. A. O'Toole, Microbial biofilms, ASM Press, 2004 Search PubMed.
- K. M. Thormann, S. Duttler, R. M. Saville, M. Hyodo, S. Shukla, Y. Hayakawa and A. M. Spormann, J. Bacteriol., 2006, 188, 2681–2691 CrossRef CAS PubMed.
- O. Bretschger, A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua and M. F. Romine, Appl. Environ. Microbiol., 2007, 73, 7003–7012 CrossRef CAS PubMed.
Footnote |
| † Weihua Guo and Shuai Luo are equally contributed. |
| This journal is © The Royal Society of Chemistry 2015 |