An effective dipping method for coating activated carbon catalyst on the cathode electrodes of microbial fuel cells
Abstract
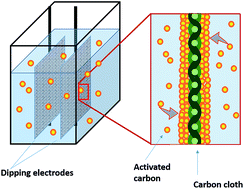
Activated carbon (AC) has been demonstrated as a promising cathode catalyst for microbial fuel cells (MFCs). A simple and effective dipping method was developed and examined for coating AC with better control of the loading rate than the commonly used brushing method.

 Please wait while we load your content...
Please wait while we load your content...