Significantly improved de/rehydrogenation properties of lithium borohydride modified with hexagonal boron nitride†
Guoping Tu,
Xuezhang Xiao,
Teng Qin,
Yiqun Jiang,
Shouquan Li,
Hongwei Ge and
Lixin Chen*
State Key Laboratory of Silicon Materials, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: lxchen@zju.edu.cn; Fax: +86 571 8795 1152; Tel: +86 571 8795 1152
First published on 3rd June 2015
Abstract
The remarkable hydrogen de/absorption properties of lithium borohydride are achieved by mechanically milling LiBH4 with hexagonal boron nitride (h-BN). It is found that the dehydrogenation properties of LiBH4 are improved with increasing the amount of h-BN. The 30 mol% h-BN doped LiBH4 composite starts to release hydrogen from just 180 °C, which is 100 °C lower than the onset dehydrogenation temperature of ball milled LiBH4. Moreover, the 30 mol% h-BN doped LiBH4 composite can release 12.6 wt% hydrogen in 2 h at 400 °C, while only 0.98 wt% H2 is gained from ball milled LiBH4. The apparent activation energy (Ea) of hydrogen desorption had been reduced from 198.31 kJ mol−1 for ball milled LiBH4 to 155.8 kJ mol−1 for 30 mol% h-BN doped LiBH4. In addition, the rehydrogenation of the composite is achieved under 400 °C and 10 MPa of H2. These remarkable results are largely attributed to the lone pair electrons of nitrogen induced destabilization of LiBH4 and their heterogeneous nucleation.
1. Introduction
Lithium borohydride (LiBH4) has been widely investigated as a candidate for hydrogen storage materials due to its large hydrogen capacity (13.8 wt%) and volumetric hydrogen density (121 kg m−3).1–3 The decomposition of LiBH4 undergoes multiple stages,4 including polymorphic transformation at about 110 °C, melting at about 280 °C with a slight accompanying hydrogen desorption, and then the main evolution of hydrogen at 400–600 °C.5,6 This major decomposition reaction is:7| LiBH4 → LiH + B + 3/2H2 | (1) |
Consequently, the desorption temperature of pristine LiBH4 is higher than the desired value. Therefore, it is worthwhile to study strategies to reduce the thermodynamic stability and the hydrogen desorption kinetics of LiBH4.
Since Züttel et al.1 reported that LiBH4 doped with SiO2 desorbs hydrogen below 200 °C, a lower temperature than for bulk LiBH4. Various potential dopants/catalysts, including metals, metal halides, and metal oxides, have been tentatively added into LiBH4 to enhance its hydrogen storage properties. The metal, such as Mg,8 Al,9 Ni,10 Ti, V, Cr and Sc,11 did not greatly improve the dehydrogenation properties of LiBH4, indicating a temperature requirement higher than 400 °C for fast hydrogen desorption and slow dehydrogenation kinetics. Taking Al doped with LiBH4 for example, 7.2 wt% hydrogen was released at 450 °C, and completing the dehydrogenation would take above 30 hours.9,11,12 For the metal halides doped LiBH4, the dehydrogenation temperature was considerably decreased to 220–300 °C by doped with FeCl2, CoCl2, NiCl2,13 LaCl3, CeCl3, LaF3 and CeF3,14 and even to about 100 °C by mixing with TiF3,15 TiCl3 (ref. 16) and ZnF2.17 However by-product including harmful diborane also will release, and thus results in drastically degraded reversible hydrogen capacity.18 By doping with metal oxides, such as Fe2O3, V2O5, Nb2O5, TiO2,19 LiBH4 was able to dehydrogenate at much lower temperatures.20–22 For example, 6 wt% hydrogen could be released at 200 °C for a LiBH4–Fe2O3 mixture with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.20 Unfortunately the destabilization of LiBH4 by metal oxides resulted from a redox reaction, which caused LiBH4 could not be rehydrogenated under moderate conditions.20
2.20 Unfortunately the destabilization of LiBH4 by metal oxides resulted from a redox reaction, which caused LiBH4 could not be rehydrogenated under moderate conditions.20
In this paper, the hydrogen storage properties of LiBH4 defined by doping with h-BN were investigated. We observed that the dehydrogenation properties of LiBH4 are improved by doping with h-BN. It is found that h-BN addition greatly facilitates the dehydrogenation kinetics of LiBH4, and the rehydrogenation properties are also enhanced. This improvement may be correlated to the lone pair electrons of nitrogen induced destabilization of LiBH4 and a heterogeneous nucleation process of the solid decomposition products of reaction (1) on the surface of BN.
2. Experimental section
Commercial hexagonal boron nitride (99%, Aladdin), and LiBH4 (95%, Acros Organics) were used in this research without further purification. Samples of LiBH4 and x h-BN (x = 0, 0.05, 0.15, 0.3, 0.5) were ball milled under a hydrogen pressure of 3 MPa for 3 h, The ball milling process was paused for 6 min every 24 min to avoid an increasing temperature of the samples. The as-synthesized samples were dehydrogenated at 400 °C under 0.1 MPa H2 and rehydrogenated at 400 °C under 10 MPa of H2. All experimental operations were performed in a high-pure Ar-filled glovebox.The X-ray diffraction (XRD) analysis of the samples were performed on an X'Pert Pro (PANalytical, Netherlands) with Cu Kα radiation at 40 kV and 40 mA with the step size of 0.02° from 10° to 90° (2θ). Scanning electron microscopy (SEM, Hitachi SU-70) was performed to examine surface morphology of the samples. The microstructure was further examined by transmission electron microscopy (TEM, JEOL JEM-1200EX working at 200 kV) and high resolution transmission electron microscopy (HRTEM, FEI Tecnai G2 F20 S-Twin working at 200 kV). The differential scanning calorimetry and mass spectrometer (DSC-MS) measurements were conducted on a synchronous thermal analysis (Netzsch STA 449F3 analyzer/Netzsch Q403C mass spectrometer) with a heating rate of 5 K min−1 from room temperature to 500 °C under high purity argon condition with a purge rate of 50 ml min−1. During the experiments the MS signals at m/z = 2, 17, and 27 were recorded to detect possible evolving gases H2, NH3 and B2H6. Hydrogen desorption and absorption properties of the samples were quantitatively examined with volumetric method by a carefully calibrated Sievert's type apparatus. In order to compare with the hydrogen capacity of bulk LiBH4, the hydrogen contents presented in this work are calculated based only on the LiBH4.
3. Results and discussion
Fig. 1(a) shows the XRD patterns of pristine and ball milled h-BN. For both the pristine h-BN and ball milled h-BN, all the detected peaks can be assigned to the diffraction of h-BN phase according to JCPDS 34-0421. The sharp and intensity of diffraction peaks in Fig. 1(a) indicate the good crystallinity of h-BN as-received sample. However, after ball milling at 400 rpm for 3 h, the diffraction peaks changed slightly, exhibited a little lower intensity and wider diffraction peaks. These may be ascribed to some amorphousization of h-BN happened, such as BN layers drifted during ball milling. The graphite-like structure of h-BN was studied by TEM and HRTEM, shown in Fig. 1(b) and (c). The TEM micrograph shows that the h-BN sample has a clear and flat surface. In the HRTEM image, the measured lattice fringes of ≈0.326 nm coincides with the lattice spacing of the (002) planes of the h-BN. The corresponding fast Fourier transform (FFT) analysis reveals hexagonal spots, indicating the structure of h-BN is hexagonal sheets.23Fig. 2 illustrates isothermal dehydrogenation curves of ball milled LiBH4 and LiBH4 doped with various amount of h-BN samples. The dehydrogenation properties of LiBH4 were markedly improved upon ball milling with h-BN, even addition amount of h-BN is only 5 mol% of LiBH4, the dehydrogenation behavior is quite different form pure LiBH4. Obviously, it could be found that the improvement effect on dehydrogenation properties of LiBH4 increases with addition amount of h-BN. When the h-BN doping amounts add up to 30 mol% of LiBH4, 12.6 wt% hydrogen can be released within 2 h at 400 °C, while only 0.98 wt% hydrogen can be gained for ball milled LiBH4. More importantly, ball milled LiBH4 finishes the decomposition will take about 6000 min, and it can be shorted to 180 min after doping with 30 mol% h-BN. Therefore, it can be concluded that the doped h-BN greatly enhanced the dehydrogenation kinetics of LiBH4.
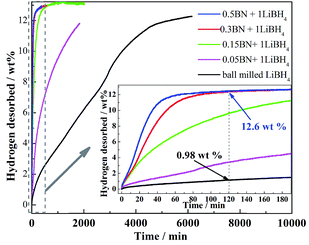 | ||
| Fig. 2 (a) Isothermal dehydrogenation curves of ball milled LiBH4 and LiBH4 doped with different amount of h-BN at 400 °C. | ||
It is common knowledge that h-BN has very good chemical inertness, especially its resistance to acids and molten metals and its stability in air up to 1000 °C.23,24 And h-BN does not react with LiBH4 neither after ball milling at 400 rpm nor after dehydrogenation at 400 °C. It is confirmed by the XRD patterns of LiBH4 doped with h-BN samples, as shown in Fig. 3. The diffraction peaks of the samples after ball milling are corresponding to starting materials (LiBH4 and h-BN), and after dehydrogenation the diffraction peaks are assigned to h-BN and LiH. LiH is the product of LiBH4 decomposition.
 | ||
| Fig. 3 XRD patterns of ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples: (a) after ball milling, (b) after dehydrogenation at 400 °C. | ||
The dehydrogenation property of the ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples were investigated by DSC-MS as shown in Fig. 4. As displayed in the DSC profiles, both ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples exhibit of four endothermic peaks during the heating process to 500 °C at 5 °C min−1. The first endothermic peak around 113 °C corresponds to the phase transition of LiBH4, and the second one around 285 °C is from melting of LiBH4. The other two peaks at higher temperatures (350–500 °C) are attributed to the decomposition of LiBH4 with hydrogen releases.1 Obviously, after doping with h-BN, the melting of LiBH4 shifted to the lower temperature (276 °C), which may be due to LiBH4 particle size reduces after ball milling with low density h-BN. The major dehydrogenation temperature of LiBH4 reduces to 431 °C, lower than ball milled LiBH4. From the MS profiles, only hydrogen was detected from the thermal desorption measurement by MS (data of possible B2H6, and NH3 are shown in Fig. 4). The main hydrogen release peak are 467 °C and 435 °C for ball milled LiBH4 and LiBH4 doped with h-BN samples respectively, which is corresponding to the DSC results. With a careful analysis, we found that LiBH4 begin releasing hydrogen at 180 °C, which is 100 °C lower than the onset dehydrogenation temperature of ball milled LiBH4. We attribute the lower onset desorption temperature of LiBH4 to the lone pair electrons of nitrogen atom on the h-BN surface, which may induce destabilization of LiBH4 by the lone pair electrons complex with “electron-deficient” molecule LiBH4.25
 | ||
| Fig. 4 DSC-MS profiles of ball milled LiBH4 and 30 mol% doped h-BN LiBH4 samples at a heating rate of 5 K min−1. | ||
In order to gather better insight of the improvement on dehydrogenation kinetics of 30 mol% h-BN doped LiBH4, apparent activation energy (Ea) related to the dehydrogenation of LiBH4 was quantitatively determined by using the Kissinger's method according to reaction (2):26
| ln(β/Tp2) = −Ea/RTp + ln(AR/Ea) | (2) |
 | ||
| Fig. 5 Kissinger's plots obtained from the DSC data for the dehydrogenation of ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples. | ||
The products of ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples after dehydrogenation were subsequently rehydrogenated at 400 °C under 10 MPa H2 for 12 h. And the XRD patterns of rehydrogenation samples are presented in Fig. 6(a). The diffraction peaks of LiBH4 doped with 30 mol% h-BN rehydrogenated samples are assigned to LiBH4, h-BN and LiH, indicating that LiBH4 was regenerated upon hydrogenation, even though the reverse reaction proceeded incompletely, due to the sequaration of a part of LiH and boron in the material after dehydrogenation.27 However, without doping with h-BN, the diffraction peaks of LiBH4 cannot be found in the rehydrogenation sample under the same condition, but some unknown and LiH diffraction peaks could be detected. The rehydrogenation of LiBH4 has been reported only under harsh conditions such as 600 °C and 35 MPa H2.29,30 Meanwhile the rehydrogenation capacity of h-BN doped LiBH4 sample after rehydrogenation at 400 °C under 10 MPa H2 for 12 h was performed, as seen in Fig. 6(b). It could be found that over 7.0 wt% hydrogen could be released in the second dehydrogenation for the 30 mol% h-BN doped LiBH4 sample. And the rehydrogenation capacity of the composites is about 6 wt% during the third dehydrogenation. In other words, experimental results of this work showed that the rehydrogenation properties of LiBH4 were improved by h-BN addition in terms of rehydrogenation conditions and rehydrogenation percentage than that of ball milled LiBH4.
 | ||
| Fig. 6 XRD patterns and isothermal dehydrogenation curves of ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples after rehydrogenation under 400 °C and 10 MPa of H2 for 12 h. | ||
In the SEM examinations of ball milled LiBH4 and 30 mol% h-BN doped LiBH4 samples, appreciable difference on the LiBH4 particle morphology was observed between ball milled LiBH4 and LiBH4 doped with h-BN. As shown in Fig. 7(a), ball milled LiBH4 particles are not small. Even after ball milling for 3 h, the particles size is over 2 μm, because of the agglomerations reunited after ball milling. Interestingly, after ball milling with 30 mol% h-BN, LiBH4 particles become much smaller than 2 μm, dispersing on h-BN flat surface, as shown in Fig. 7(c). It is believed that decreasing particle size is beneficial to enhance dehydrogenation of LiBH4.31,32 Fig. 7(b) and (f) show the SEM images of ball milled LiBH4 and 30 mol% doped LiBH4 after dehydrogenation at 400 °C. Obviously, after dehydrogenation, the ball milled LiBH4 decomposition product (B and LiH) particles agglomerated together to form larger clusters. For 30 mol% h-BN doped LiBH4 sample, the decomposition product particles size does not increase. In this regard, the h-BN additives might play a role as effect heterogeneous nucleation site for the decomposition and prevent the nucleated particles from further growth during dehydrogenation reaction.33 These might be largely credible explanation for why the dehydrogenation and rehydrogenation properties of LiBH4 are improved by doping with h-BN.
Based on the above analyses, the whole process of synthesis and de/rehydrogenation for LiBH4 doped with h-BN systems from a micro point of view is illustrated in Scheme 1. After LiBH4 ball milled with h-BN sheets, LiBH4 particles become small and well disperse on the surface of h-BN. The lone pair electrons of N atom on the surface of h-BN induces part of LiBH4 destabilization, resulting in the onset of dehydrogenation of LiBH4 was decreased to 180 °C with a small amount of hydrogen desorption, which also could be supported by the dehydrogenation of LiBH4 doped with graphite as shown in the ESI Fig. S2† During the dehydrogenation process, the LiBH4 covered on the surface of h-BN will decomposes firstly, and then the products of LiBH4 decomposition (LiH and B particles) play the role of the nucleation sites. And with effect heterogeneous nucleation site of h-BN, the product particles of LiBH4 decomposition are more inclined to dispersing on the surface of h-BN. In the rehydrogenation process, the composites can reverse to form LiBH4 in short distance on the surface of h-BN.
 | ||
| Scheme 1 Illustration of the process of synthesis and de/rehydrogenation for LiBH4 doped with h-BN systems. | ||
4. Conclusions
The effects of h-BN additives on the dehydrogenation and rehydrogenation properties of LiBH4 were investigated. The results show that the dehydrogenation kinetics of LiBH4 is greatly improved by doping with h-BN. For instance, 30 mol% h-BN doped LiBH4 can release 12.6 wt% hydrogen in 2 h at 400 °C, while only 0.98 wt% H2 is gained for ball milled LiBH4. The onset dehydrogenation temperature of LiBH4 is reduced to 180 °C from 280 °C of ball milled LiBH4, the apparent activation energy of hydrogen desorption has been reduced from 198.31 kJ mol−1 for ball milled LiBH4 to 155.8 kJ mol−1 for LiBH4 doped with 30 mol% h-BN. Meanwhile, over 7.0 wt% hydrogen can be rehydrogenated at 400 °C under 10 MPa H2. XRD, SEM and TEM analyses demonstrate that h-BN remain stable in the ball milling process and de/rehydrogenation. It is believed that h-BN dopant services as effect heterogeneous nucleation site for dehydrogenation of LiBH4 and plays a role in preventing the nucleated particles from further growth. Besides, the presupposition complex reaction between the lone pair electrons of N atom on surface of h-BN with “electron-deficient” molecule LiBH4 induces destabilization of LiBH4 might take effect on improving the dehydrogenation of LiBH4.Acknowledgements
The authors gratefully acknowledge the financial supports for this research from the National High Technology Research & Development Program of China (2012AA051503), the National Natural Science Foundation of China (51471151, 51171173), the Program for Innovative Research Team in University of Ministry of Education of China (IRT13037), and the Zhejiang Provincial Science & Technology Program of China (2014C31134).References
- A. Züttel, S. Rentsch, P. Fischer, P. Wenger, P. Sudan, P. Mauron and C. Emmenegger, J. Alloys Compd., 2003, 356, 515–520 CrossRef.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- A. Züttel, A. Borgschulte and S. I. Orimo, Scr. Mater., 2007, 56, 823–828 CrossRef PubMed.
- Y. Yan, A. Remhof, S.-J. Hwang, H.-W. Li, P. Mauron, S.-i. Orimo and A. Züttel, Phys. Chem. Chem. Phys., 2012, 14, 6514–6519 RSC.
- F. Pendolino, P. Mauron, A. Borgschulte and A. Züttel, J. Phys. Chem. C, 2009, 113, 17231–17234 CAS.
- P. Ngene, P. Adelhelm, A. M. Beale, K. P. de Jong and P. E. de Jongh, J. Phys. Chem. C, 2010, 114, 6163–6168 CAS.
- A. Züttel, P. Wenger, S. Rentsch, P. Sudan, P. Mauron and C. Emmenegger, J. Power Sources, 2003, 118, 1–7 CrossRef.
- J. F. Mao, Z. Wu, X. B. Yu, T. Don, T. J. Chen, B. C. Weng, J. Ni, N. X. Xu and T. S. Huang, Rare Met. Mater. Eng., 2007, 36, 2248–2250 CAS.
- M. Meggouh, D. M. Grant and G. S. Walker, J. Phys. Chem. C, 2011, 115, 22054–22061 CAS.
- J. Xu, Y. Li, J. Y. Cao, R. R. Meng, W. C. Wang and Z. D. Chen, Catal. Sci. Technol., 2015, 5, 1821–1828 CAS.
- J. Yang, A. Sudik and C. Wolverton, J. Phys. Chem. C, 2007, 111, 19134–19140 CAS.
- G. L. Xia, X. B. Yu and Z. Wu, Rare Met. Mater. Eng., 2009, 38, 1618–1621 CAS.
- B. J. Zhang and B. H. Liu, Int. J. Hydrogen Energy, 2010, 35, 7288–7294 CrossRef CAS PubMed.
- B. J. Zhang, B. H. Liu and Z. P. Li, J. Alloys Compd., 2011, 509, 751–757 CrossRef CAS PubMed.
- Y. H. Guo, X. B. Yu, L. Gao, G. L. Xia, Z. P. Guo and H. K. Liu, Energy Environ. Sci., 2010, 3, 465–470 CAS.
- T. Sun, H. Wang, Q. G. Zhang, D. L. Sun, X. D. Yao and M. Zhu, J. Mater. Chem., 2011, 21, 9179–9184 RSC.
- Z. G. Zhang, H. Wang and M. Zhu, Int. J. Hydrogen Energy, 2011, 36, 8203–8208 CrossRef CAS PubMed.
- H.-W. Li, Y. Yan, S.-i. Orimo, A. Züttel and C. M. Jensen, Energies, 2011, 4, 185–214 CrossRef CAS PubMed.
- H. Liu, L. Jiao, Y. Zhao, K. Cao, Y. Liu, Y. Wang and H. Yuan, J. Mater. Chem. A, 2014, 2, 9244–9250 CAS.
- X. B. Yu, D. M. Grant and G. S. Walker, J. Phys. Chem. C, 2009, 113, 17945–17949 CAS.
- X. B. Yu, D. A. Grant and G. S. Walker, J. Phys. Chem. C, 2008, 112, 11059–11062 CAS.
- M. Q. Fan, L. X. Sun, Y. Zhang, F. Xu, J. Zhang and H. L. Chu, Int. J. Hydrogen Energy, 2008, 33, 74–80 CrossRef CAS PubMed.
- J. Greim and K. A. Schwetz, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, DOI:10.1002/14356007.a04_295.pub2.
- I. Smith and R. P. Lerner, Folia Primatol., 1971, 14, 110–117 CrossRef CAS PubMed.
- J. P. Soulie, G. Renaudin, R. Cerny and K. Yvon, J. Alloys Compd., 2002, 346, 200–205 CrossRef CAS.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- P. A. Ward, J. A. Teprovich, B. Peters, J. Wheeler, R. N. Compton and R. Zidan, J. Phys. Chem. C, 2013, 117, 22569–22575 CAS.
- J. Shao, X. Z. Xiao, X. L. Fan, L. T. Zhang, S. Q. Li, H. W. Ge, Q. D. Wang and L. X. Chen, J. Phys. Chem. C, 2014, 118, 11252–11260 CAS.
- S. Orimo, Y. Nakamori, G. Kitahara, K. Miwa, N. Ohba, S. Towata and A. Züttel, J. Alloys Compd., 2005, 404–406, 427–430 CrossRef CAS PubMed.
- P. Mauron, F. Buchter, O. Friedrichs, A. Remhof, M. Bielmann, C. N. Zwicky and A. Züttel, J. Phys. Chem. B, 2008, 112, 906–910 CrossRef CAS PubMed.
- M. Christian and K. F. Aguey-Zinsou, Nanoscale, 2010, 2, 2587–2590 RSC.
- X. F. Wan and L. L. Shaw, Acta Mater., 2011, 59, 4606–4615 CrossRef CAS PubMed.
- Z. Z. Fang, X. D. Kang and P. Wang, Int. J. Hydrogen Energy, 2010, 35, 8247–8252 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DSC curves with different rates and isothermal dehydrogenation curves of the LiBH4 doped with graphite. See DOI: 10.1039/c5ra05438a |
| This journal is © The Royal Society of Chemistry 2015 |


