Mesoporous NaYF4:Yb,Er@Au–Pt(iv)-FA nanospheres for dual-modal imaging and synergistic photothermal/chemo-anti-cancer therapy†
Abstract
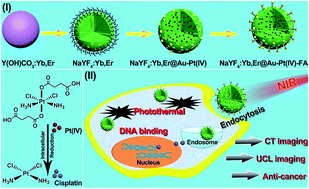
In this report, mesoporous NaYF4:Yb,Er@Au–Pt(IV)-FA up-conversion nanoparticles (UCNPs) have been designed by attaching Au NPs and Pt(IV) pro-drugs on the surface of PEI hydrogel modified mesoporous NaYF4:Yb,Er nanospheres. Finally the molecules modified with folic acid (FA) improve the receptor-mediated endocytosis. Because of the doped rare earth ions in the host matrix, the as-synthesized platform exhibits excellent up-conversion luminescence (UCL) imaging and computed X-ray tomography (CT) imaging properties. Diverse methods including MTT assay, hemolysis experiments, and live/dead cell analysis were employed to evaluate the biocompatibility and ablation efficacy of the as-synthesized platform. It was found that the cytotoxicity of the platform can be tuned by eliminating the axial ligands reductively during intracellular endocytosis. Especially, under 980 nm near-infrared (NIR) irradiation, the platform shows excellent inhibition toward cancer cells due to the synergistic photothermal injury to enzymes and membrane integrity combined with the DNA binding of activated Pt(II) to avoid cell proliferation. The developed nanocomposite may thus be a promising imaging-guided synergistic anti-cancer platform.

 Please wait while we load your content...
Please wait while we load your content...