Study of FePt deposited reduced graphene oxide's utility as a catalyst towards oxygen reduction and methanol oxidation reactions†
Abstract
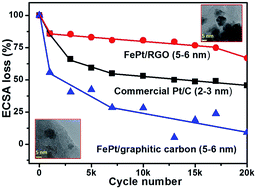
Hydrogen and methanol fuelled polymer electrolyte fuel cell's (PEFC) penetration in the commercial market is slowed by the use of expensive Pt and PtRu as electrocatalysts. Transition metal based Pt alloy catalysts have historically struggled for durability in acidic environments. Reduced graphene oxide (RGO) supported Pt alloy catalysts have gained significant interest recently due to improvements in catalyst–support interaction that lead to better durability and performance. In this report we investigate the performance and durability aspects of FePt supported on RGO towards oxygen reduction and methanol oxidation reactions. PXRD and TEM results show that the FePt nanoparticle size is in the range of 4–7 nm and TGA measurements show that the metal loading of the catalyst is ∼55%. Electrochemical measurements towards ORR reveal a significant improvement in activity and durability for FePtGO over commercial PtC and FePtC. The utilization of RGO as a support certainly increases the lifetime of transition metal–Pt alloys that are generally susceptible to durability issues under acidic environments in fuel cells.

 Please wait while we load your content...
Please wait while we load your content...