Obtaining a novel crystalline/amorphous core/shell structure in barium titanate nanocrystals by an innovative one-step approach
Abstract
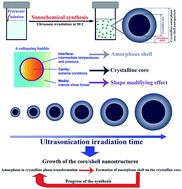
The synthesis temperature and purity of core/shell nanocrystals are key challenges facing the scientific community. Moreover, core/shell structures usually need a multi-step synthesis pathway to be fabricated. This work aims to address these challenges by developing an innovative one-step synthesis pathway for obtaining carbonate-free BaTiO3 core/shell nanocrystals at low temperature. The results show that very fine BaTiO3 core/shell nanocrystals (<11 nm) free from any by-products such as BaTi2O5 and BaCO3 are synthesized at 323 K (50 °C). This newly developed structure can significantly reduce the sintering temperature of the nanocrystals and can be beneficial in fabricating the ceramic parts at lower sintering temperatures.

 Please wait while we load your content...
Please wait while we load your content...