Design of a solar-driven TiO2 nanofilm on Ti foil by self-structure modifications†
Yan Liu‡a,
Kangsheng Mu‡a,
Jiandan Zhonga,
Keni Chena,
Yanzong Zhang
*ab,
Gang Yangab,
Lilin Wangab,
Shihuai Dengab,
Fei Shenab and
Xiaohong Zhangab
aCollege of Environment, Sichuan Agricultural University, Chengdu, Sichuan 611130, China. E-mail: yzzhang@sicau.edu.cn
bSichuan Engineering and Technical Center for Rural Environmental Protection, Sichuan Agricultural University, Chengdu, Sichuan 611130, China
First published on 15th April 2015
Abstract
A novel solar-driven TiO2 nanofilm (VO–N–TiO2 (A/R) nanofilm), which was obtained by self-structure modifications, was designed on a Ti substrate surface. The VO–N–TiO2 (A/R) nanofilm showed a three-dimensional pine branched-like structure and a disordered lattice structure, which consisted of VO, N-doping and an anatase/rutile phase, was built in the VO–N–TiO2 (A/R) nanofilm. The experimental results show that the band gap of the VO–N–TiO2 (A/R) nanofilm can be narrowed to 0.72 eV and 2.4 eV and that it exhibited excellent optical absorption capacity in the 200–2500 nm region. Using the VO–N–TiO2 (A/R) nanofilm to degrade methyl orange, the removal efficiency could reach 96% within 150 min under sunlight, which shows its high photoelectrocatalytic activity and stability.
1 Introduction
Titanium dioxide (TiO2) is one of the most promising multifunctional materials that can be used in extensive applications such as photocatalysis, air purification, photovoltaic cells, solar cells, and water splitting.1–3 However, TiO2 can only be excited under UV irradiation (<387.5 nm) because of its large band gap (anatase of 3.2 eV and rutile of 3.0 eV).4 The introduction of dopants and coupling with narrow band gap semiconductors have been carried out to extend the optical absorption into the visible-light region.5–7 N-doping of TiO2 is considered as the optimal method to improve its photocatalytic activity under visible light.8,9 N-doped TiO2 was first synthesized by sputtering a TiO2 target in an N2 (40%)/Ar gas mixture, and this can extend the optical absorption to 550 nm.10 Recently, a N-doped TiO2 film was prepared using the anodization process, and this film also has a photoresponse under visible light.11 In addition, crystal phase of TiO2, morphology, and disorder structure consisting Ti3+ and oxygen vacancy (VO) have been proved to be effective approaches in improving its photochemical activity.12 The mixed-phase of TiO2 displays an enhanced photocatalytic activity due to the rapid transfer of electrons from rutile to anatase on the TiO2 interface.13,14 VO-doped TiO2 shows considerable potential in extending the visible light absorption.15,16 The band gap of TiO2 can be narrowed to 1.85 eV by the synergistic effect of VO and surface disorder.17 Its optical absorption can be further extended to 1200 nm as a result of introducing a disordered structure in the nanophase TiO2 surface using the hydrogenation process.18 Recent research has been carried out to investigate the fundamental properties and photocatalytic applications of hydrogenated black TiO2.19,20In this study, we design a novel solar-driven TiO2 nanofilm (VO–N–TiO2 (A/R) nanofilm) by adopting a new electrolyte system in the anodic oxidation process. A three-dimensional pine-bough-like structure nanofilm that has a disordered lattice structure, consisting of VO, N-doping and an anatase/rutile phase, was obtained. These variations in structure were defined as “self-structural modification”. The optical absorption of the VO–N–TiO2 (A/R) nanofilm by self-structural modification covered the ultraviolet, visible and near-infrared (NIR) region from 200 nm to 2500 nm and displayed improved photoelectrocatalytic activity under sunlight.
2 Experimental
2.1 Synthesis of VO–N–TiO2 (A/R) nanofilm
Titanium foil (thickness 0.2 mm, purity 99.6%, Baoji Titanium Nickel Gold Port Equipment Manufacturing Co., Ltd.) was clipped to the size of 3.3 cm × 4 cm, then ultrasonically degreased in acetone and ethyl alcohol, and rinsed in distilled water for 15 min. The Ti sheets were chemically polished in a mixed solution of HF (40%), HNO3 (65%) and distilled water (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for 20 s and then rinsed in distilled water. The Ti sheet was used as the anode, and Pt foil was used as the counter electrode. Anodization was performed in an ethylene glycol solution containing HNO3 (1 wt%) and water (1 wt%) at 40 V for 10 min using a DC power supply at room temperature. The samples were cleaned and dried and then annealed at 773 K for 1 h in a muffle furnace. The annealed samples were designated as VO–N–TiO2 (A/R) nanofilm.
5) for 20 s and then rinsed in distilled water. The Ti sheet was used as the anode, and Pt foil was used as the counter electrode. Anodization was performed in an ethylene glycol solution containing HNO3 (1 wt%) and water (1 wt%) at 40 V for 10 min using a DC power supply at room temperature. The samples were cleaned and dried and then annealed at 773 K for 1 h in a muffle furnace. The annealed samples were designated as VO–N–TiO2 (A/R) nanofilm.
2.2 Characterization
The morphology of the samples was observed using field-emission scanning electron microscopy (FE-SEM, S-4800, Hitachi). Crystalline structures were investigated by glancing angle X-ray diffraction (GLXRD) using Cu-Kα (0.15418 nm) radiation at 40 kV and 30 mA from 20° to 80° at 0.03° step and a glancing angle of 1°. Raman measurement was recorded in the range of 100–1000 cm−1 using a Horiba Jobin Yvon LabRAM HR800 with 532 nm laser excitation, and the power of the sample was estimated to be 5 mW and the spectral resolution of the spectrometer was 1 cm−1. The chemical state of the elements was evaluated using X-ray photoelectron spectroscopy (XPS, Perkin-Elmer Corporation, Eden Prairie, MN) with an Mg K anode (1253.6 eV photon energy, 15 kV, 300 W) at a takeoff angle of 45°. The binding energy values were calibrated using the contaminant carbon (C 1s = 284.4 eV) as a reference. Multiplex XPS spectra of Ti 2p, O 1s, and N 1s were recorded using the band-pass energy of 35.75 eV, which corresponds to an energy resolution of 1.2 eV. Atomic concentrations of these elements were obtained by comparing the peak areas of their spectra. The VO identification was carried out using electron spin resonance spectroscopy (ESR, JES FA-200). The settings were as follows: 323.717 mT center field, 323.717 ± 25 mT sweep width, 9077.574 mHz microwave frequency, 100.00 kHz modulation amplitude, and 3.0 mW power. UV-vis-NIR absorption spectra were obtained using a TU-1901 spectrometer with the wavelength range of 200 nm–2500 nm. Photoluminescence (PL) spectra were obtained using a fluorescence spectrophotometer (FLsp 920) with excitation wavelengths of 280 and 354 nm.2.3 Electrochemical performance
Photoelectrochemical properties were evaluated using an electrochemical workstation (CHI 830C Instruments, China) in the standard three-electrode configuration. A 0.5 M Na2SO4 solution was used as the electrolyte. The VO–N–TiO2 (A/R) nanofilm was used as the working electrode with an active area of 1 cm2, and Pt foil and Ag|AgCl were used as the counter and reference electrodes, respectively. The I–t curve was used to evaluate the photocurrent density. Light irradiation intensity was recorded using an illuminometer (TES-1339).2.4 Photoelectrocatalytic activity
The photoelectrocatalytic activity of the VO–N–TiO2 (A/R) nanofilm was evaluated by the decomposition of methyl orange (MO) under sunlight (MO concentration 20 mg L−1, pH value 6.8 and volume 40 mL). A bias voltage of 4 V (the effect of different bias potentials on MO degradation see ESI Fig. S1†) was applied during the MO decomposition. The solution absorbance was measured using a UV-vis spectrophotometer (UV3000, China) at 464 nm. The decomposition rate was calculated using eqn (1),
 | (1) |
3 Results and discussion
3.1 Morphologies and crystal structure
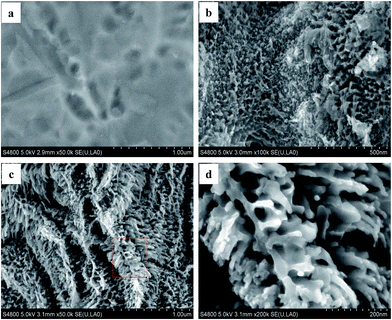
Irregular holes could be observed on the Ti substrate surface after chemical polishing (Fig. 1a). The flocculent structures were formed on the Ti substrate surface when the voltage of 40 V was applied to the two electrodes for 10 min (Fig. 1b). Interestingly, a three-dimensional pine-bough-like structure nanofilm was obtained after calcination at 773 K for 1 h in oxic atmosphere (Fig. 1c), and the pine-bough-like nanoclusters consisted of many nano-sized branches grown on the bole (Fig. 1d).The as-anodized sample presented an amorphous structure, whereas the VO–N–TiO2 (A/R) nanofilm showed a mixed crystal structure of rutile and anatase (Fig. 2a). The mixed crystal parameters were calculated using Scherrer's equation21 (Table 1) and were different from their theoretical value. This is due to the disordered lattice structure, which was caused by the reconstruction of the anatase/rutile interfaces.22 Moreover, the following peaks were observed in the Raman spectrum (Fig. 2b). Eg (a) ∼ 146 cm−1, Eg (a) ∼ 197 cm−1, B1g (a) ∼ 399 cm−1, A1g (a) + B1g (a) ∼ 517 cm−1, and Eg (a) ∼ 639 cm−1 which were assigned to anatase.18,23,24 Eg (r) ∼ 442 cm−1 and A1g (r) ∼ 619 cm−1 were assigned to rutile.23,25 Clearly, the two complementary methods, XRD and Raman spectroscopy, revealed that the as-synthesized TiO2 film was composed of an anatase and rutile phase.
 | ||
| Fig. 2 (a) XRD patterns for the as-anodized sample and VO–N–TiO2 (A/R) nanofilm and (b) Raman scattering pattern of the VO–N–TiO2 (A/R) nanofilm. | ||
| Annealing | 2-Theta (°) | Phase ID | Area | FWHM | Crystallite size (nm) | Lattice parameters | Cell volume (Å3) | |
|---|---|---|---|---|---|---|---|---|
| a = b | c | |||||||
| Unannealed | — | Amorphous | — | — | — | — | — | — |
| Annealed | 25.45 | Anatase | 566 | 0.401 | 16.01 | 3.785 | 9.514 | 142.86 |
| 27.39 | Rutile | 680 | 0.495 | 16.24 | 4.953 | 2.959 | 72.59 | |
3.2 Chemical state of the elements
The VO–N–TiO2 (A/R) nanofilm contained Ti, O, N, and C (Fig. 3a). The amount of N is ∼3.79 at%, which is higher than that mentioned in previous reports.11,26,27 The C element was introduced from residual organic carbons.28 The peaks at 458.47 and 464.12 eV were attributed to Ti4+ from Ti 2p3/2 and 2p1/2, respectively (Fig. 3b).29 However, the binding energy of Ti 2p3/2 shifted ∼0.3 eV towards a lower binding energy, which shows the presence of VO and a few N species.30,31 The peak at 399.6 eV was attributed to the interstitial nitrogen dopant32,33 (Fig. 3c). The peak at 407.2 eV originated from the nitrate species in the anodization process.34,35 The peak at 529.8 eV was attributed to O–Ti–O in the TiO2 crystal lattice (Fig. 3d), whereas the peak at 531.6 eV was assigned to the hydroxyl species.16,36 | ||
| Fig. 3 (a) Full XPS spectra of the VO–N–TiO2 (A/R) nanofilm, (b) multiplex high-resolution scan over Ti 2p, (c) N 1s, (d) O 1s, and (e) low temperature ESR spectra of the VO–N–TiO2 (A/R) nanofilm. | ||
The signal at g = 2.003 in the ESR spectrum (Fig. 3e) confirmed the presence of VO in the VO–N–TiO2 (A/R) nanofilm,37 and the concentration of VO was approximately 1.0 × 1015. N doping always leads to thermal instability in TiO2 and thus increases VO formation.38,39
3.3 Formation mechanism of the VO–N–TiO2 (A/R) nanofilm
Based on the abovementioned experimental results and analysis, the formation mechanism of the VO–N–TiO2 (A/R) nanofilm, which contains three stages, was proposed.At the first stage, nitric ions are forced to accumulate at the titania/solution interface under an electric field and attack the oxide layer on the surface of the Ti substrate to form a soluble complex such as [TiO2−x(NO3)x]m−n (m > n, 0 < x < 2), which leads to the local dissolution/thinning of the oxide layer and the penetration of the oxide layer or irregular holes into the Ti substrate (Fig. S2†).40 Similarly, the accumulated nitric anions form a soluble complex such as [Ti(NO3)n]m−n (m = 3, 4; n > 4) in concurrence with the formation of titania, which results in pore generation and growth along the electric field direction. Nevertheless, the complex anions [Ti(NO3)n]m−n are thermodynamically unstable and further decompose through electrochemical reactions into the thermodynamically stable TiO2 and interstitial nitrogen dopant.40 Moreover, the NO species derived from the decomposition of nitric acid could be chemisorbed on the surface or incorporated into the lattice of the resultant TiO2 (ref. 41) as indicated by XPS. The formation of flocculent structure films on the Ti substrate is dependent on the anodic oxidation rate of titanium and the anodic dissolution rate of the resultant titania in the electrolyte. Under the current conditions, the dynamic equilibrium of the anodization-dissolution rate led to the uniform growth of flocculent structures.
At the second stage, the flocculent structure film is further dehydrated, crystallized and expanded during calcination. The anatase-to-rutile transformation was conducted at the wide temperature range of 673–1373 K.42 The rutile phase evolved mainly from the inner regions of the agglomerated TiO2 particles, and the anatase phase emerged in the surface region during the phase transformation because of the asymmetric heat dissipation at the interface.43,44 The Vo defects increase with the increase in the annealing temperature, and N doping can increase VO formation.42,45 In addition, the interface between the Ti substrate and oxide layer may react as Ti + TiO2 → TiOx (x = 0–2), which may form more VO.46 Such VO can ulteriorly promote the anatase-to-rutile transformation, because it involves the overall shrinkage of the oxygen structure and a cooperative movement of ions.47,48 Due to the different thermal expansion coefficients of anatase (10.2 × 10−6 K−1) and rutile (7.14 × 10−6 K−1),49 the outer anatase layer grew faster than the inner rutile core during calcinations. The flocculent structures ultimately evolved into three-dimensional pine-bough-like structures after calcination for 1 h.
At the last stage, fast cooling is key process for the formation of a disordered structure and VO defects. The metastable defective phase on the surface such as VO can subsequently become nearly stoichiometric when the sample is calcined at high temperatures and is quickly exposed to oxygen-rich air.17 This means that the VO defects on the surface immediately decreases and reaches a steady state in oxygen-rich conditions,50 but the VO defects still maintains a high level in the internal lattice on the lower surface.
3.4 Optical absorption
Ti foil almost has no optical absorption (Fig. 4a). However, the optical absorption of the VO–N–TiO2 (A/R) nanofilm covered the ultraviolet, visible and near-infrared (NIR) region from 200 nm to 2500 nm. One of the absorption edges was extended to 475 nm. The extension of light absorption from UV to visible light is attributed to the localized states near the valence band (VB), which is induced by interstitial nitrogen atoms.8,11 Another huge absorption peak appeared at ∼830 nm and extended to 2500 nm, which was due to the disordered lattice structure that was introduced by the anatase/rutile interface and VO.14,17,18 The multi-peaks in the absorbance spectra indicated that the band gap of the VO–N–TiO2 (A/R) nanofilm was drastically narrowed by intra-band transitions.1 The band gap was evaluated by the plots of (Ahv)1/2 vs. excitation energy (hv).29 Fig. 4b shows that the band gaps were narrowed to 0.72 eV and 2.4 eV. | ||
| Fig. 4 (a) UV-vis-NIR absorption spectra of the VO–N–TiO2 (A/R) nanofilm and Ti foil, and (b) the variation of (Ahv)1/2 vs. excitation energy (hv) for the VO–N–TiO2 (A/R) nanofilm. | ||
To further examine the electronic structure and defect levels of the VO–N–TiO2 (A/R) film, we analyzed the valence band (VB) edge spectra, which were obtained using XPS and PL emission spectroscopy. As shown in Fig. 5a, the typical value of the VB maximum (VMB) for TiO2 NTs was located at ∼1.2 eV below the Fermi energy.18 However, for the VO–N–TiO2 (A/R) film, the VMB was located at ∼0.4 eV, and the band tail continuously extended to −0.28 eV. That is, a blue shift about 1.48 eV toward the vacuum level was observed, which indicated the presence of extra states above the VB.18,51
The VB edge at ∼0.4 eV was introduced by the N 2p band level located at 0.8 eV above the VB.31,52,53 As observed in the PL emission spectra (Fig. S3(a)†), the peaks at 515 nm (2.4 eV) and 561 nm (2.2 eV) correspond to the band energy from the N 2p to conduction band (CB) for anatase and rutile.54,55 Surface disorder, which is induced by hydrogenation, can induce a substantial shift of 2.18 eV above the VB position,18 and that caused by carbon doping can cause a shift of 1.6 eV toward the vacuum level.51 Core–shell black TiO2 with the fast cooling step also induces a substantial shift of 1.5 eV above the VBM.17 In our case, a number of states, which were caused by the presence of interstitial N atoms and interface disorder of anatase/rutile, were possible for a maximum energy of 1.48 eV above the VBM of VO–N–TiO2 (A/R).
Furthermore, the high concentration of VO can create shallow levels at 0.7–1.0 eV below the CBM.17,56,57 Serried peaks from 506 nm (2.45 eV) to 590 nm (2.1 eV) were observed in the PL emission spectra (Fig. S3(b)†), which were assigned to the recombination of defective states from the VO to VB for anatase and rutile.58 In addition, the peaks at 437 nm (2.8 eV) and 456.7 nm (2.7 eV) can be ascribed to self-trapped excitons located at the TiO6 octahedra.29 Based on the abovementioned analysis, a schematic of the band alignment model from the VB to CB was constructed and is shown in Fig. 5b, and a schematic of the electronic transitions in different energy levels is summarized in Fig. 6.
 | ||
| Fig. 6 Schematic of the electronic transitions in different energy levels in the VO–N–TiO2 (A/R) nanofilm. | ||
The CB edge of TiO2 (A) is about 0.2 eV higher than that of TiO2 (R).59 All of the excitation energies from (1) to (14) existed in the composite system. The excited wavelength and energy gap for each excited channel are listed in Table 2. Only channel (1) needs to be excited by UV light irradiation (<388 nm). Channels (2) to (6) can be excited by visible light (<721 nm), and (7) to (14) can even be excited by near-infrared light (<1824 nm).
| Channels | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eg (eV) | 3.2 | 3.0 | 2.4 | 2.2 | 2.2 | 1.72 | 1.52 | 1.48 | 1.4 | 1.0 | 0.8 | 0.8 | 0.72 | 0.68 |
| λ (nm) | 388 | 413 | 517 | 564 | 564 | 721 | 816 | 838 | 886 | 1240 | 1550 | 1550 | 1722 | 1824 |
3.5 Electrochemical performance
The photocurrent density of the VO–N–TiO2 (A/R) nanofilm was approximately 20 μA cm−2 under solar irradiation, whereas that of the Ti foil was almost zero (Fig. 7). The illumination intensity of sunlight and temperature are shown in Table 3.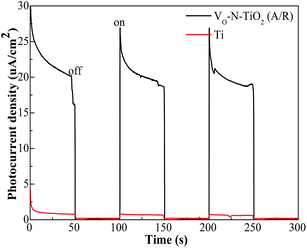 | ||
| Fig. 7 Photocurrent density of the VO–N–TiO2 (A/R) nanofilm and Ti foil measured at zero bias voltage versus Ag|AgCl in 0.5 M Na2SO4 aqueous solution. | ||
| Degradation time (min) | 30 | 60 | 90 | 120 | 150 |
|---|---|---|---|---|---|
| Illumination intensity (lx) | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 045 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
| Temperature (K) | 283 | 283 | 284 | 284 | 283 |
The removal efficiency of MO reached 96% for VO–N–TiO2 (A/R), whereas the control sample was only 66% within 150 min under sunlight (Fig. 8a). In addition, neither the photocatalysis process (PC, 0 V bias) nor the electrocatalysis process (EC, with 4 V bias in the dark) could effectively decompose MO. Therefore the photoelectrocatalysis (PEC) process exhibited a notable synergistic effect. The photoelectrocatalytic activity did not exhibit any reduction after 10 cycles (Fig. 8b), indicating the exceptional stability of the VO–N–TiO2 (A/R) nanofilm in the PEC process.
 | ||
| Fig. 8 (a) Solar-driven photoelectrocatalytic activity of the VO–N–TiO2 (A/R) nanofilm and pure TiO2 (control sample); (b) cycling tests of the VO–N–TiO2 (A/R) nanofilm. | ||
4 Conclusions
The VO–N–TiO2 (A/R) nanofilm exhibits significant potential in high-efficiency utilization of solar energy. The high photoelectrocatalytic activity of the VO–N–TiO2 (A/R) nanofilm under sunlight was attributed to three main facts. (1) The distortion of the anatase/rutile interface with VO and N atom doping caused a disordered lattice structure, narrowed the band gap of TiO2, promoted the optical absorption from 200 nm to 2500 nm, and interfacial electron transfer. (2) The unique three-dimensional pine-bough-like structures increased the surface area of the nanofilm, providing more adsorptive and active sites, meanwhile promoting the light adsorption of scattering and diffuse reflection. (3) The photo-generated electron–hole pairs can be separated further by applying a bias voltage.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by Program for Changjiang Scholars and Innovative Research Team in University (IRT13083) from the Ministry of Education of China.References
- X. Chen, L. Liu, Y. Y. Peter and S. S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- G. Wang, H. Wang, Y. Ling, Y. Tang, X. Yang, R. C. Fitzmorris, C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026–3033 CrossRef CAS PubMed.
- W. Guo, F. Zhang, C. Lin and Z. L. Wang, Adv. Mater., 2012, 24, 4761–4764 CrossRef CAS PubMed.
- L. Cui, Y. Wang, M. Niu, G. Chen and Y. Cheng, J. Solid State Chem., 2009, 182, 2785–2790 CrossRef CAS PubMed.
- J. Xue, Q. Shen, W. Liang, X. Liu and F. Yang, Electrochim. Acta, 2013, 97, 10–16 CrossRef CAS PubMed.
- H. Xie, W. Que, Z. He, P. Zhong, Y. Liao and G. Wang, J. Alloys Compd., 2013, 550, 314–319 CrossRef CAS PubMed.
- C. W. Lai and S. Sreekantan, Int. J. Hydrogen Energy, 2013, 38, 2156–2166 CrossRef CAS PubMed.
- J. Wang, D. N. Tafen, J. P. Lewis, Z. Hong, A. Manivannan, M. Zhi, M. Li and N. Wu, J. Am. Chem. Soc., 2009, 131, 12290–12297 CrossRef CAS PubMed.
- R. Nakamura, T. Tanaka and Y. Nakato, J. Phys. Chem. B, 2004, 108, 10617–10620 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, science, 2001, 293, 269–271 CrossRef CAS PubMed.
- K. Shankar, K. C. Tep, G. K. Mor and C. A. Grimes, J. Phys. D: Appl. Phys., 2006, 39, 2361 CrossRef CAS.
- L. Liu and X. Chen, Chem. Rev., 2014, 114, 9890–9918 CrossRef CAS PubMed.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, J. Phys. Chem. B, 2003, 107, 4545–4549 CrossRef CAS.
- X. Zhang, Y. Lin, D. He, J. Zhang, Z. Fan and T. Xie, Chem. Phys. Lett., 2011, 504, 71–75 CrossRef CAS PubMed.
- V. N. Kuznetsov and N. Serpone, J. Phys. Chem. C, 2009, 113, 15110–15123 CAS.
- J. Zhuang, S. Weng, W. Dai, P. Liu and Q. Liu, J. Phys. Chem. C, 2012, 116, 25354–25361 CAS.
- A. Naldoni, M. Allieta, S. Santangelo, M. Marelli, F. Fabbri, S. Cappelli, C. L. Bianchi, R. Psaro and V. Dal Santo, J. Am. Chem. Soc., 2012, 134, 7600–7603 CrossRef CAS PubMed.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- B. Chen, J. A. Beach, D. Maurya, R. B. Moore and S. Priya, RSC Adv., 2014, 4, 29443–29449 RSC.
- N. Liu, C. Schneider, D. Freitag, M. Hartmann, U. Venkatesan, J. Müller, E. Spiecker and P. Schmuki, Nano Lett., 2014, 14, 3309–3313 CrossRef CAS PubMed.
- Q. Wang, X. Yang, X. Wang, M. Huang and J. Hou, Electrochim. Acta, 2012, 62, 158–162 CrossRef CAS PubMed.
- N. A. Deskins, S. Kerisit, K. M. Rosso and M. Dupuis, J. Phys. Chem. C, 2007, 111, 9290–9298 CAS.
- T. Xia, N. Li, Y. Zhang, M. B. Kruger, J. Murowchick, A. Selloni and X. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 9883–9890 CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- T. Lan, X. Tang and B. Fultz, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 094305 CrossRef.
- D. Kim, S. Fujimoto, P. Schmuki and H. Tsuchiya, Electrochem. Commun., 2008, 10, 910–913 CrossRef CAS PubMed.
- S. Hoang, S. Guo, N. T. Hahn, A. J. Bard and C. B. Mullins, Nano Lett., 2011, 12, 26–32 CrossRef PubMed.
- Y. C. Zhang, M. Yang, G. Zhang and D. D. Dionysiou, Appl. Catal., B, 2013, 142–143, 249–258 CrossRef CAS PubMed.
- B. Santara, P. K. Giri, K. Imakita and M. Fujii, J. Phys. Chem. C, 2013, 117, 23402–23411 CAS.
- V. Etacheri, M. K. Seery, S. J. Hinder and S. C. Pillai, Adv. Funct. Mater., 2011, 21, 3744–3752 CrossRef CAS PubMed.
- G. Liu, H. G. Yang, X. Wang, L. Cheng, J. Pan, G. Q. Lu and H.-M. Cheng, J. Am. Chem. Soc., 2009, 131, 12868–12869 CrossRef CAS PubMed.
- P. Roy, S. Berger and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 2904–2939 CrossRef CAS PubMed.
- Z. Wang, W. Cai, X. Hong, X. Zhao, F. Xu and C. Cai, Appl. Catal., B, 2005, 57, 223–231 CrossRef CAS PubMed.
- X. Chen and C. Burda, J. Am. Chem. Soc., 2008, 130, 5018–5019 CrossRef CAS PubMed.
- S.-K. Joung, T. Amemiya, M. Murabayashi and K. Itoh, Appl. Catal., A, 2006, 312, 20–26 CrossRef CAS PubMed.
- R. Li, H. Kobayashi, J. Guo and J. Fan, Chem. Commun., 2011, 47, 8584–8586 RSC.
- J. Dong, J. Han, Y. Liu, A. Nakajima, S. Matsushita, S. Wei and W. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 1385–1388 CAS.
- M. Batzill, E. H. Morales and U. Diebold, Phys. Rev. Lett., 2006, 96, 026103 CrossRef.
- C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi and E. Giamello, J. Phys. Chem. B, 2005, 109, 11414–11419 CrossRef CAS PubMed.
- S. Chu, S. Inoue, K. Wada, S. Hishita and K. Kurashima, J. Electrochem. Soc., 2005, 152, B116–B124 CrossRef CAS PubMed.
- K. Hadjiivanov and H. Knözinger, Phys. Chem. Chem. Phys., 2000, 2, 2803–2806 RSC.
- D. A. Hanaor and C. C. Sorrell, J. Mater. Sci., 2011, 46, 855–874 CrossRef CAS.
- J. Zhang, M. Li, Z. Feng, J. Chen and C. Li, J. Phys. Chem. B, 2006, 110, 927–935 CrossRef CAS PubMed.
- W. Junwei, M. Ashish Kumar, Z. Qing and H. Liping, J. Phys. D: Appl. Phys., 2013, 46, 255303 CrossRef.
- V. Sivaram, E. J. Crossland, T. Leijtens, N. K. Noel, J. Alexander-Webber, P. Docampo and H. J. Snaith, J. Phys. Chem. C, 2014, 118, 1821–1827 CAS.
- D. Fang, Z. Luo, K. Huang and D. C. Lagoudas, Appl. Surf. Sci., 2011, 257, 6451–6461 CrossRef CAS PubMed.
- C. Rath, P. Mohanty, A. Pandey and N. Mishra, J. Phys. D: Appl. Phys., 2009, 42, 205101 CrossRef.
- R. D. Shannon and J. A. Pask, J. Am. Ceram. Soc., 1965, 48, 391–398 CrossRef CAS PubMed.
- S. Li, J. Chen, F. Zheng, Y. Li and F. Huang, Nanoscale, 2013, 5, 12150–12155 RSC.
- S. Wendt, P. T. Sprunger, E. Lira, G. K. H. Madsen, Z. Li, J. Ø. Hansen, J. Matthiesen, A. Blekinge-Rasmussen, E. Lægsgaard, B. Hammer and F. Besenbacher, Science, 2008, 320, 1755–1759 CrossRef CAS PubMed.
- S. Kurian, H. Seo and H. Jeon, J. Phys. Chem. C, 2013, 117, 16811–16819 CAS.
- Y. Wang, C. Feng, M. Zhang, J. Yang and Z. Zhang, Appl. Catal., B, 2010, 100, 84–90 CrossRef CAS PubMed.
- M. Harb, P. Sautet and P. Raybaud, J. Phys. Chem. C, 2011, 115, 19394–19404 CAS.
- W. Zhang, M. Zhang, Z. Yin and Q. Chen, Appl. Phys. B: Lasers Opt., 2000, 70, 261–265 CrossRef CAS.
- J. L. Sumerel, W. Yang, D. Kisailus, J. C. Weaver, J. H. Choi and D. E. Morse, Chem. Mater., 2003, 15, 4804–4809 CrossRef CAS.
- F. Zuo, L. Wang, T. Wu, Z. Zhang, D. Borchardt and P. Feng, J. Am. Chem. Soc., 2010, 132, 11856–11857 CrossRef CAS PubMed.
- E. Finazzi, C. Di Valentin, G. Pacchioni and A. Selloni, J. Chem. Phys., 2008, 129, 154113 CrossRef PubMed.
- Q. Xiang, K. Lv and J. Yu, Appl. Catal., B, 2010, 96, 557–564 CrossRef CAS PubMed.
- T. Kawahara, Y. Konishi, H. Tada, N. Tohge, J. Nishii and S. Ito, Angew. Chem., 2002, 114, 2935–2937 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra05367f |
| ‡ These authors contributed equally to this work (co-first authors). |
| This journal is © The Royal Society of Chemistry 2015 |