Cobalt(II) complexes with the quinolone antimicrobial drug oxolinic acid: structure and biological perspectives†
Evgenia P. Irgia,
George D. Geromichalosb,
Sofia Balalac,
Jakob Kljund,
Stavros Kalogiannisc,
Athanasios Papadopoulosc,
Iztok Tureld and
George Psomas*a
aDepartment of General and Inorganic Chemistry, Faculty of Chemistry, Aristotle University of Thessaloniki, GR-54124 Thessaloniki, Greece. E-mail: gepsomas@chem.auth.gr; Fax: +30 2310997738; Tel: +30 2310997790
bCell Culture, Molecular Modeling and Drug Design Lab, Symeonidion Research Center, Theagenion Cancer Hospital, Thessaloniki GR-54007, Greece
cDepartment of Nutrition and Dietetics, Faculty of Agriculture, Food Technology and Nutrition, Alexander Technological Educational Institution, Sindos, Thessaloniki, Greece
dFaculty of Chemistry and Chemical Technology, University of Ljubljana, Vesna pot 113, 1000 Ljubljana, Slovenia
First published on 13th April 2015
Abstract
The interaction of cobalt(II) with the quinolone antimicrobial agent oxolinic acid (Hoxo) in the absence or presence of the Lewis bases 2,2′-bipyridine (bipy), 2,2′-bipyridylamine (bipyam), 1,10-phenanthroline (phen), pyridine (py) or 4-benzylpyridine (4bzpy) resulted in the formation of a series of mononuclear complexes which were characterized with physicochemical and spectroscopic techniques. The crystal structure of [Co(oxo)2(bipy)]·3MeOH was determined by X-ray crystallography. The interaction of the complexes with calf-thymus DNA (CT DNA) was investigated by UV spectroscopy, viscosity measurements and cyclic voltammetry in order to evaluate the possible DNA-binding mode and to calculate the corresponding DNA-binding constants. The binding of the complexes to human or bovine serum albumin was monitored by fluorescence emission spectroscopy and relatively high binding constant values were determined. The antimicrobial activity of the complexes was tested against four different microorganisms (Escherichia coli, Xanthomonas campestris, Staphylococcus aureus and Bacillus subtilis) and was found to be similar to that of free Hoxo. Molecular docking simulations on the crystal structure of CT DNA, HSA and BSA were employed in order to study in silico the ability of the resultant complexes to bind biomacromolecules. This is the first report for metal–quinolone complexes combining experimental data and molecular docking simulations of their interaction with DNA and serum albumins.
Introduction
Quinolones are synthetic antibacterial drugs which are widely used in the treatment of urinary tract, respiratory and bone-joint infections as well as sexually transmitted diseases, prostatitis, pneumonia and acute bronchitis.1–4 Their main mode of action is the inhibition of DNA topoisomerase II, one of the most important enzymes involved in the replication and transcription of DNA.5,6 The study of the biological properties of metal complexes7 with quinolones as ligands includes the binding to DNA8–10 and serum albumin proteins11–14 and the evaluation of the antibacterial4,15–17 and the antiproliferative activity.18,19Oxolinic acid (Hoxo, Scheme 1(A)) is a first-generation quinolone antimicrobial drug3 which has been used for the treatment of urinary tract infections for almost five decades.2,20 Nevertheless, only five of its complexes have been structurally characterized; a Cu(II),21 two Zn(II),22 a Ni(II)12 and a Mn(II)23 oxolinato complex, all reported by our group.7
The most important role of cobalt in vivo is its presence in the active center of vitamin B12, which participates indirectly in the regulation of the DNA synthesis.24 Cobalt is also involved in the co-enzyme of vitamin B12 provided as a supplement of the vitamin,25 while eight cobalt-dependent proteins have been reported.24 Since the first reports of the biological activity of a cobalt compound sixty years ago,26 diverse structurally characterized cobalt complexes showing antioxidant,27,28 antiproliferative,29,30 antiviral,31,32 bactericidal,33,34 and fungicidal35,36 activity have been reported. Concerning the cobalt–quinolone complexes, six Co(II) complexes with ciprofloxacin,37 enoxacin,38 enrofloxacin,39 norfloxacin and sarafloxacin40 as ligands have been structurally characterized.
In order to investigate the potential synergism between metal ions and quinolones,7 we have synthesized cobalt(II) complexes with oxolinic acid in the presence of the oxygen-donor methanol, or a series of nitrogen-donor ligands such as the N-donor ligands pyridine (py) or 4-benzylpyridine (4bzpy) or the N,N′-donors 2,2′-bipyridine (bipy), 2,2′-bipyridylamine (bipyam) or 1,10-phenanthroline (phen) (Scheme 1(B)–(F)). The synthesis, characterization and structure of the complexes [Co(oxo)2(MeOH)2] (1), [Co(oxo)2(bipy)]·3MeOH (2·3MeOH), [Co(oxo)2(bipyam)] (3), [Co(oxo)2(phen)] (4), [Co(oxo)2(py)2] (5) and [Co(oxo)2(4bzpy)2] (6) are presented herein. The crystal structure of [Co(oxo)2(bipy)]·3MeOH (2·3MeOH) was determined by X-ray crystallography. Our studies of the biological properties of complexes 1–6 were focused on: (i) the interaction of the complexes with calf-thymus (CT) DNA which was investigated by UV spectroscopy, cyclic voltammetry, and viscosity measurements in order to elucidate the binding mode and to determine the binding strength to DNA, (ii) the ability of the complexes to displace ethidium bromide (EB), a classical DNA–intercalator, from its DNA–EB complex as an indirect way to verify the existence of potential intercalation, which was monitored by fluorescence emission spectroscopy, (iii) the ability of the complexes to bind to proteins involved in their potential transport through the bloodstream,41 such as bovine (BSA) and human (HSA) serum albumins, investigated by fluorescence spectroscopy, (iv) their antimicrobial activity evaluated by determining the half-minimum inhibitory concentration (IC50) and the minimum inhibitory concentration (MIC) against the microorganisms Escherichia coli NCTC 29212 (E. coli), Xanthomonas campestris ATCC 1395 (X. campestris), Staphylococcus aureus ATCC 6538 (S. aureus) and Bacillus subtilis ATCC 6633 (B. subtilis) and (v) in silico approaches with the employment of molecular docking simulations on the crystal structure of CT DNA and the target serum albumin proteins, HSA and BSA, which were used with the aim to explore the ability of complexes 1–6 to bind to these biomacromolecules.
Experimental
Materials and instrumentation
Oxolinic acid, CoCl2·6H2O, bipy, phen, bipyam, py, 4bzpy, KOH, NaCl, trisodium citrate, CT DNA, BSA, HSA and EB were purchased from Sigma-Aldrich Co. and all solvents were purchased from Merck. All the chemicals and solvents were reagent grade and were used as purchased. TEAP was recrystallized twice from ethanol, prior to its use, and dried under vacuum.DNA stock solution was prepared by dilution of CT DNA to buffer (containing 15 mM trisodium citrate and 150 mM NaCl at pH 7.0) followed by exhaustive stirring for three days, and kept at 4 °C for no longer than a week. The stock solution of CT DNA gave a ratio of UV absorbance at 260 and 280 nm (A260/A280) in the range of 1.8–1.9, indicating that the DNA was sufficiently free of protein contamination.42 The DNA concentration was determined by the UV absorbance at 260 nm after 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dilution using ε = 6600 M−1 cm−1.43
20 dilution using ε = 6600 M−1 cm−1.43
Infrared (IR) spectra (400–4000 cm−1) were recorded on a Nicolet FT-IR 6700 spectrometer with samples prepared as KBr disk. UV-visible (UV-vis) spectra were recorded as nujol mulls and in solution at concentrations in the range 10−5 to 5 × 10−3 M on a Hitachi U-2001 dual beam spectrophotometer. Room temperature magnetic measurements were carried out on a magnetic susceptibility balance of Sherwood Scientific (Cambridge, UK) by the Faraday method. C, H and N elemental analysis were performed on a Perkin-Elmer 240B elemental analyzer. Molar conductivity measurements of 1 mM DMSO solutions of the complexes were carried out with a Crison Basic 30 conductometer. Fluorescence spectra were recorded in solution on a Hitachi F-7000 fluorescence spectrophotometer. Viscosity experiments were carried out using an ALPHA L Fungilab rotational viscometer equipped with an 18 mL LCP spindle.
Cyclic voltammetry studies were performed on an Eco chemie Autolab Electrochemical analyzer. Cyclic voltammetry experiments were carried out in a 30 mL three-electrode electrolytic cell. The working electrode was platinum disk, a separate Pt single-sheet electrode was used as the counter electrode and a Ag/AgCl electrode saturated with KCl was used as the reference electrode. The cyclic voltammograms of the complexes in solution were recorded at ν = 100 mV s−1. All electrochemical measurements were performed at 25.0 ± 0.2 °C.
Synthesis of the complexes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1636 (vs (very strong)); νasym(CO2), 1586 (vs); νsym(CO2), 1396 (vs); Δ = νasym(CO2) − νsym(CO2): 190 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 730 (sh (shoulder)), 545, 448, 405 (sh), 335, 322; in DMSO: 736 (sh) (25), 533 (45), 455 (sh) (25), 410 (sh) (180), 337 (4800), 325 (4500). μeff at room temperature = 4.19 BM. The complex is soluble in ethanol, acetonitrile, acetone, DMF and DMSO (ΛM = 6 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1636 (vs (very strong)); νasym(CO2), 1586 (vs); νsym(CO2), 1396 (vs); Δ = νasym(CO2) − νsym(CO2): 190 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 730 (sh (shoulder)), 545, 448, 405 (sh), 335, 322; in DMSO: 736 (sh) (25), 533 (45), 455 (sh) (25), 410 (sh) (180), 337 (4800), 325 (4500). μeff at room temperature = 4.19 BM. The complex is soluble in ethanol, acetonitrile, acetone, DMF and DMSO (ΛM = 6 S cm2 mol−1, in 1 mM DMSO solution).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1386 (vs); Δ = 202 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 555, 468, 405 (sh), 339, 321; in DMSO: 733 (sh) (25), 560 (60), 475 (sh) (45), 405 (sh) (210), 337 (6000), 325 (5700). μeff at room temperature = 4.38 BM. The complex is soluble in DMF and DMSO (ΛM = 12 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1386 (vs); Δ = 202 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 555, 468, 405 (sh), 339, 321; in DMSO: 733 (sh) (25), 560 (60), 475 (sh) (45), 405 (sh) (210), 337 (6000), 325 (5700). μeff at room temperature = 4.38 BM. The complex is soluble in DMF and DMSO (ΛM = 12 S cm2 mol−1, in 1 mM DMSO solution).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1635 (vs); νasym(CO2), 1589 (vs); νsym(CO2), 1386 (vs); Δ = 203 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 555, 448 (sh), 408 (sh), 338, 322; in DMSO: 743 (sh) (20), 559 (40), 450 (sh) (30), 410 (sh) (150), 337 (6500), 322 (8900). μeff at room temperature = 4.35 BM. The complex is soluble in methanol, ethanol, acetonitrile, DMF and DMSO (ΛM = 10 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1635 (vs); νasym(CO2), 1589 (vs); νsym(CO2), 1386 (vs); Δ = 203 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 555, 448 (sh), 408 (sh), 338, 322; in DMSO: 743 (sh) (20), 559 (40), 450 (sh) (30), 410 (sh) (150), 337 (6500), 322 (8900). μeff at room temperature = 4.35 BM. The complex is soluble in methanol, ethanol, acetonitrile, DMF and DMSO (ΛM = 10 S cm2 mol−1, in 1 mM DMSO solution).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1393 (vs); Δ = 195 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 745 (sh), 550, 445 (sh), 407 (sh), 338, 325; in DMSO: 741 (sh) (15), 545 (30), 455 (sh) (120), 411 (sh) (320), 336 (7500), 328 (6900). μeff at room temperature = 4.40 BM. The complex is soluble in DMF and DMSO (ΛM = 10 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1393 (vs); Δ = 195 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 745 (sh), 550, 445 (sh), 407 (sh), 338, 325; in DMSO: 741 (sh) (15), 545 (30), 455 (sh) (120), 411 (sh) (320), 336 (7500), 328 (6900). μeff at room temperature = 4.40 BM. The complex is soluble in DMF and DMSO (ΛM = 10 S cm2 mol−1, in 1 mM DMSO solution).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1395 (vs); Δ = 193 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 742 (sh), 555, 455 (sh), 413 (sh), 337, 322; in DMSO: 745 (sh) (15), 557 (35), 460 (sh) (65), 415 (sh) (290), 338 (3800), 326 (3500). μeff at room temperature = 4.25 BM. The complex is soluble in DMF and DMSO (ΛM = 8 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1634 (vs); νasym(CO2), 1588 (vs); νsym(CO2), 1395 (vs); Δ = 193 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 742 (sh), 555, 455 (sh), 413 (sh), 337, 322; in DMSO: 745 (sh) (15), 557 (35), 460 (sh) (65), 415 (sh) (290), 338 (3800), 326 (3500). μeff at room temperature = 4.25 BM. The complex is soluble in DMF and DMSO (ΛM = 8 S cm2 mol−1, in 1 mM DMSO solution).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone, 1634 (vs); νasym(CO2), 1581 (vs); νsym(CO2), 1394 (vs); Δ = 187 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 552, 454 (sh), 411 (sh), 337, 325; in DMSO: 745 (sh) (20), 557 (35), 460 (sh) (65), 415 (sh) (290), 338 (4500), 326 (5500). μeff at room temperature = 4.15 BM. The complex is soluble in methanol, ethanol, DMF and DMSO (ΛM = 9 S cm2 mol−1, in 1 mM DMSO solution).
O)pyridone, 1634 (vs); νasym(CO2), 1581 (vs); νsym(CO2), 1394 (vs); Δ = 187 cm−1 (KBr disk); UV-vis: λ, nm (ε, M−1 cm−1) as nujol mull: 740 (sh), 552, 454 (sh), 411 (sh), 337, 325; in DMSO: 745 (sh) (20), 557 (35), 460 (sh) (65), 415 (sh) (290), 338 (4500), 326 (5500). μeff at room temperature = 4.15 BM. The complex is soluble in methanol, ethanol, DMF and DMSO (ΛM = 9 S cm2 mol−1, in 1 mM DMSO solution).X-ray structure determination
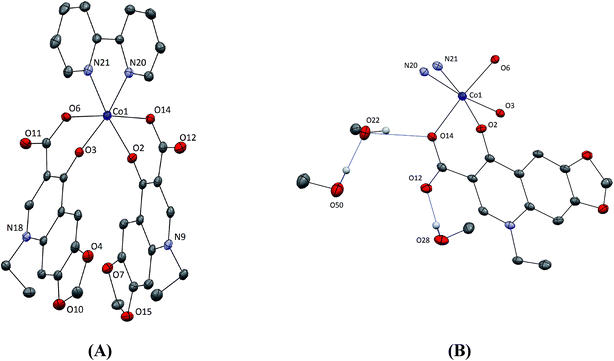
X-ray diffraction data for 2 was collected on a Nonius Kappa CCD diffractometer at 150(2) K equipped with a Mo anode (Kα radiation, λ = 0.71073 Å) and a graphite monochromator (Table S1†). The structure was solved by direct methods implemented in SIR92 (ref. 44) and refined by a full-matrix least-squares procedure based on F2 using SHELXL-97.45 All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were either placed at calculated positions and treated using appropriate riding models or determined from the difference Fourier map. The program Mercury was used for data analysis and figure preparation.46†DNA binding studies
The interaction of complexes 1–6 with CT DNA was studied with UV spectroscopy in order to investigate the possible binding modes to CT DNA and to calculate the binding constants to CT DNA (Kb). The UV spectra of CT DNA were recorded for a constant CT DNA concentration in the presence of each complex at diverse r (=[complex]/[CT DNA] mixing ratios) values. The binding constants, Kb (in M−1), of the complexes with CT DNA were determined by Wolfe–Shimer equation (eqn (S1)†)47 and plots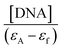 versus [DNA] using the UV spectra of the compound recorded for a constant concentration in the absence or presence of CT DNA for diverse r values. Control experiments with DMSO were also performed and no changes in the spectra of CT DNA were observed.
versus [DNA] using the UV spectra of the compound recorded for a constant concentration in the absence or presence of CT DNA for diverse r values. Control experiments with DMSO were also performed and no changes in the spectra of CT DNA were observed.
The viscosity of a DNA solution was measured in the presence of increasing amounts of the complexes. The obtained data are presented as (η/η0)1/3 versus r, where η0 and η is the viscosity measured for a DNA solution in the absence and presence of the complex, respectively, and L and L0 denote the apparent molecular length in the presence and absence of the complex, respectively.23,28
The interaction of complexes 1–6 with CT DNA has been also investigated by monitoring the changes observed in the cyclic voltammogram of a 0.40 mM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 DMSO
2 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer solution of complex upon addition of CT DNA at diverse r values. The buffer was used as the supporting electrolyte.
buffer solution of complex upon addition of CT DNA at diverse r values. The buffer was used as the supporting electrolyte.
The competitive studies of each complex with EB were investigated with fluorescence emission spectroscopy in order to investigate whether the complex can displace EB from its CT DNA–EB complex. The CT DNA–EB complex was prepared by adding 20 μM EB and 26 μM CT DNA in buffer (150 mM NaCl and 15 mM trisodium citrate at pH 7.0). The possible intercalating effect of the complexes was studied by adding a certain amount of a solution of the complex step by step into a solution of the DNA–EB complex. The influence of the addition of each complex to the DNA–EB complex solution was obtained by recording the variation of fluorescence emission spectra with excitation wavelength at 540 nm. The oxolinato complexes 1–6 do not show any fluorescence emission band at room temperature in solution or in the presence of CT DNA under the same experimental conditions (i.e. λexc = 540 nm); therefore, the observed quenching is attributed to the displacement of EB from its EB–DNA complex. The values of the Stern–Volmer constant (KSV, in M−1) were calculated according to the linear Stern–Volmer equation (eqn (S2)†)48 and the plots 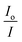 vs. [Q].
vs. [Q].
Albumin binding experiments
The protein binding study was performed by tryptophan fluorescence quenching experiments using bovine (BSA, 3 μM) or human serum albumin (HSA, 3 μM) in buffer (containing 15 mM trisodium citrate and 150 mM NaCl at pH 7.0). The quenching of the fluorescence emission of tryptophan residues of BSA at 342 nm or HSA at 351 nm with an excitation wavelength at 295 nm was monitored using complexes 1–6 as quenchers with increasing concentration.49 The oxolinato complexes 1–6 in buffer solutions exhibited under the same experimental conditions a low-intensity emission band at 365 nm. Therefore, the quantitative studies of the SA fluorescence emission spectra were performed after their correction by subtracting the spectra of the complexes. The influence of the inner-filter effect on the measurements was evaluated by eqn (S3).†50 The Stern–Volmer and Scatchard equations (eqn (S4)–(S6)†)51 and graphs were used in order to study the interaction of each quencher with SAs and calculate the dynamic quenching constant KSV (in M−1), the approximate quenching constant kq (in M−1 s−1), the association binding constant K (in M−1) and the number of binding sites per albumin n.Determination of minimum inhibitory concentration
The antimicrobial activity of Hoxo and its complexes 1–6 was evaluated by determining their respective half-minimum inhibitory concentration (IC50) and the minimum inhibitory concentration (MIC) values towards four bacterial species, namely two Gram negative (E. coli NCTC 29212 and X. campestris ATCC 1395) and two Gram positive (S. aureus ATCC 6538 and B. subtilis ATCC 6633). Cultures of these microbial strains were grown on a rich selective agar medium and stored at 4 °C. The selective media used were Nutrient Agar or broth for B. subtilis and S. aureus, Yeast Mold Agar or Broth for X. campestris and Luria Agar or Broth for E. coli. Cells picked from the surface of the stored cultures were used to initiate liquid pre-cultures of the same selective medium at an initial turbidity of roughly 1 McFarland unit. Pre-cultures were incubated for 24 h in a rotary shaking incubator and subsequently they were used to inoculate the test cultures used for the determination of MIC at an initial turbidity of 0.5 McFarland units. The test cultures consisted of Mueller–Hinton broth (Deben Diagnostics Ltd) containing different concentrations of the compounds. Different concentrations were achieved as follows: the compounds were freshly dissolved in DMSO to a concentration of 1 mg mL−1 and they were diluted with DMSO, using the method of progressive double dilution. Thus working solutions with decreasing concentrations of the compounds under investigation were achieved. The working solutions were subsequently diluted to the final desired concentration by addition to the growth medium at a proportion of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98. MIC values were determined as the lowest concentrations of the tested compounds that inhibited visible growth of each respective organism after a 24 h incubation.52 Bacterial growth was determined by measuring the turbidity of appropriately diluted cultures at 600 nm with reference to equally diluted sterile growth medium and the inhibition achieved was calculated by comparing the turbidity of each culture to the average of the turbidity of three non-inhibited cultures. The IC50 values were calculated using linear regression equation of the inhibitory effects of at least three concentrations equal or higher than the MIC and the decadic logarithm of the concentration.53 All test cultures were grown in triplicates and for the determination of MIC, growth had to be inhibited in at least two cultures of the triplicate. Incubation temperature at all stages was 37 °C except for X. campestris that was cultivated at 28 °C.
98. MIC values were determined as the lowest concentrations of the tested compounds that inhibited visible growth of each respective organism after a 24 h incubation.52 Bacterial growth was determined by measuring the turbidity of appropriately diluted cultures at 600 nm with reference to equally diluted sterile growth medium and the inhibition achieved was calculated by comparing the turbidity of each culture to the average of the turbidity of three non-inhibited cultures. The IC50 values were calculated using linear regression equation of the inhibitory effects of at least three concentrations equal or higher than the MIC and the decadic logarithm of the concentration.53 All test cultures were grown in triplicates and for the determination of MIC, growth had to be inhibited in at least two cultures of the triplicate. Incubation temperature at all stages was 37 °C except for X. campestris that was cultivated at 28 °C.
In silico computational methods (molecular modelling & docking calculations)
We used the determined X-ray crystallographic structure of CT DNA (PDB accession number 1BNA) and the target proteins HSA (PDB accession number 2BXG) and BSA (PDB accession number 4OR0) in complex with bound co-crystallized drugs ibuprofen (IBP) for HSA and naproxen (NPS) for BSA. The chosen crystal structures have been refined at 1.9 Å resolution for the synthetic DNA dodecamer d(CpGpCpGpApApTpTpCpGpCpG),54 at 2.70 Å resolution for HSA complexed with IBP,55 and at 2.58 Å resolution for BSA complexed with NPS.56 For the docking calculations, only the A chain of the protein was used since chain B is replicate, with IBP and NPS bound at the same ligand binding site among the chains. For this reason, the data for chain B and of each drug referring to this chain were deleted from corresponding PDB files. Molecular models of the compounds were built in 3D coordinates and their best most stable (lower energy) conformation was detected by geometrical optimization of their structure in the gas phase, as implemented in the Spartan '10 Molecular Modelling program suite.57 The molecules' structures were initially optimized by conformational search using the Monte Carlo method with the MMFF94 molecular mechanics model. Geometry optimization was accomplished via quantum-chemical calculations by utilizing ab initio Hartree–Fock method with 6-31G* basis set.Docking calculations were carried out via BioMedCAChe program, which is part of the CAChe package (CAChe WorkSystem Pro version 7.5.0.85, Fujitsu). Docking experiments employed full ligand flexibility and partial DNA or protein flexibility focused at the ligand binding site. Molecular docking studies were carried out on the crystal structure of CT DNA and human and bovine serum albumins to investigate the effect of the studied compounds on these target macromolecules. X-ray structures were obtained from the Brookhaven Protein Data Bank.58 The produced compound–DNA and compound–protein complexes were ranked by the energy score, including their binding conformations. 3D models of the above macromolecule crystal structures were developed after the deletion of the co-crystallized bound compound. The docking procedure provides the treatment of ligand flexibility within the DNA or protein binding site by means of a 4-point chiral pharmacophoric comparison between the ligand and the site. The final output of the docking procedure is a set of solutions ranked according to the corresponding scoring function values, each defined by the 3D coordinates of its atoms and expressed as a PDB file. The accuracy of BioMedCAChe was shown to successfully reproduce experimentally observed binding modes, in terms of rmsd (root-mean squared deviation). BioMedCAChe provided excellent result as previously observed for the low values of rmsd between experimental and docked structures of co-crystallized drugs IBP and NPS (best docked solution 0.33 Å and 0.24 Å, respectively) (this is also shown by superimposition of the above structures). The ability to accurately predict the binding conformations of HSA and BSA bound compounds IBP and NPS gave confidence that the BioMedCAChe could also predict with a similar accuracy, the binding conformations of the studied complexes 1–6 with certain amino acids. The PyMol molecular graphics system was used in order to visualize the molecules and the results of the docking experiments.59
Results and discussion
Synthesis and characterization of the complexes
The complexes were synthesized via the aerobic reaction of oxolonic acid deprotonated by KOH with CoCl2·6H2O in the presence of the corresponding co-ligand (MeOH for 1, N,N′-donor (B = bipy, bipyam, phen) for 2–4 and N-donor (L = py or 4bzpy) for 5–6) according to the following equations:| CoCl2·6H2O + 2Hoxo + 2KOH + 2MeOH → [Co(oxo)2(MeOH)2] + 2KCl + 8H2O | (1) |
| CoCl2·6H2O + 2Hoxo + 2KOH + B → [Co(oxo)2(B)] + 2KCl + 8H2O | (2) |
| CoCl2·6H2O + 2Hoxo + 2KOH + 2L → [Co(oxo)2(L)2] + 2KCl + 8H2O | (3) |
The resultant complexes 1–6 are soluble in DMF and DMSO. The values of the molar conductivity (ΛM) of 1 mM DMSO solution of the complexes are within the range 6–12 S cm2 mol−1 and might suggest a partial ionization. Nevertheless, the dissociation degree is very low (for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte, the ΛM value is expected to be ∼70 S cm2 mol−1); therefore, we may consider that the compounds do not dissociate in DMSO solution. The complexes were characterized by elemental analysis, IR and UV-vis spectroscopic techniques, magnetic measurements at room temperature and the structure of 2 was determined by X-ray crystallography.
1 electrolyte, the ΛM value is expected to be ∼70 S cm2 mol−1); therefore, we may consider that the compounds do not dissociate in DMSO solution. The complexes were characterized by elemental analysis, IR and UV-vis spectroscopic techniques, magnetic measurements at room temperature and the structure of 2 was determined by X-ray crystallography.
IR spectroscopy confirmed the deprotonation and binding mode of oxolinic acid. In the IR spectra of complexes 1–6, the disappearance of the ν(O–H) stretching vibration of free Hoxo at 3443 (broad, medium) cm−1 was indicative of the deprotonation of the carboxylate group upon binding to metal ion. The bands at 1712 (s (strong)) cm−1 and 1260 (s) cm−1 attributed to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)carboxyl and ν(C–O)carboxyl stretching vibrations of the carboxyl group (–COOH) of free Hoxo shifted in the range 1581–1589 cm−1 and 1386–1396 cm−1 characterized as antisymmetric, νasym(C
O)carboxyl and ν(C–O)carboxyl stretching vibrations of the carboxyl group (–COOH) of free Hoxo shifted in the range 1581–1589 cm−1 and 1386–1396 cm−1 characterized as antisymmetric, νasym(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and symmetric, νsym(C
O), and symmetric, νsym(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), stretching vibrations of the carboxylato group, respectively. The values of the parameter Δ [=νasym(C
O), stretching vibrations of the carboxylato group, respectively. The values of the parameter Δ [=νasym(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) − νsym(C
O) − νsym(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)] appeared in the range 187–203 cm−1 suggesting a monodentate coordination mode of the carboxylate group.60 These bands in combination with the slightly shifted vibration band ν(C
O)] appeared in the range 187–203 cm−1 suggesting a monodentate coordination mode of the carboxylate group.60 These bands in combination with the slightly shifted vibration band ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)pyridone from 1640 cm−1 up to 1634 cm−1 suggest coordination of the deprotonated oxolinato ligands to cobalt in a chelating bidentate mode via the pyridone oxygen and a carboxylato oxygen.3,12–14
O)pyridone from 1640 cm−1 up to 1634 cm−1 suggest coordination of the deprotonated oxolinato ligands to cobalt in a chelating bidentate mode via the pyridone oxygen and a carboxylato oxygen.3,12–14
The UV-vis spectra of the complexes have been recorded as nujol mull and in DMSO solution and are similar suggesting that the complexes retain their structure in solution. In the visible region of the spectrum, three low-intensity bands have been observed at 730–745 nm (band I), 533–560 nm (band II) and at 445–475 nm (band III). These bands can be assigned to d–d transitions; more specifically, to 4T1g(F) → 4T2g, 4T2g(F) → 4A2g and 4T1g(F) → 4T1g(P) transitions, respectively. The number and the location of these bands are typical for distorted octahedral high-spin Co2+ complexes.24 Additionally, an absorption band assigned to charge-transfer transition exists at 405–415 nm as observed in previously reported metal–quinolone complexes.12–14,21–23
Furthermore, in order to explore the stability of the complexes in the presence of the buffer solution used for the biological experiments, the UV-vis spectra were also been recorded upon addition of a series of buffer solutions in the pH range 6–8 (150 mM NaCl and 15 mM trisodium citrate at pH values regulated by HCl solution). In all cases (data not shown), there were not any significant changes, i.e. shift of the λmax or new peaks, showing that the complexes keep their integrity also in the presence of the buffer solution used for the biological experiments. Therefore, complexes 1–6 are non-electrolytes in DMSO solution and have the same spectral pattern in nujol and in DMSO solution as well as in the presence of the buffer solutions; the compounds are stable in solution keeping their integrity.
The observed values of μeff (=4.15–4.40 BM) for complexes 1–6 at room temperature are higher than the spin-only value (=3.87 BM) showing spin–orbit coupling due to t2g5eg2 electron configuration. The values are within the range reported for mononuclear high-spin Co(II) complexes (S = 3/2) having a distorted octahedral geometry.24,27,28
Structure of the complexes
| Bond distance | (Å) | Bond distance | (Å) |
|---|---|---|---|
| Co(1)–O(2) | 2.094(3) | Co(1)–O(14) | 2.070(3) |
| Co(1)–O(3) | 2.079(3) | Co(1)–N(20) | 2.123(4) |
| Co(1)–O(6) | 2.032(3) | Co(1)–N(21) | 2.114(4) |
| Bond angle (°) | (°) | Bond angle | (°) |
|---|---|---|---|
| O(2)–Co(1)–O(3) | 88.11(13) | O(3)–Co(1)–O(6) | 86.91(13) |
| O(2)–Co(1)–O(6) | 91.26(14) | O(3)–Co(1)–O(14) | 91.12(14) |
| O(2)–Co(1)–O(14) | 83.36(14) | O(3)–Co(1)–N(20) | 171.19(14) |
| O(2)–Co(1)–N(20) | 99.61(14) | O(3)–Co(1)–N(21) | 95.76(15) |
| O(2)–Co(1)–N(21) | 173.22(15) | O(14)–Co(1)–N(20) | 93.96(15) |
| O(6)–Co(1)–O(14) | 174.33(14) | O(14)–Co(1)–N(21) | 90.98(15) |
| O(6)–Co(1)–N(20) | 88.68(15) | N(20)–Co(1)–N(21) | 76.99(16) |
| O(6)–Co(1)–N(21) | 94.50(15) |
The Co(II) atom is six-coordinated having a distorted octahedral geometry; four oxygen atoms from two oxolinato ligands and two nitrogen atoms from the bidentate 2,2′-bipyridine ligand occupy the six vertices of octahedron. The two pyridone oxygen atoms O(2) and O(3) are cis to each other [O(2)–Co(1)–O(3) = 88.11(13)°] and the two carboxylato oxygen atoms O(6) and O(14) are in a trans arrangement [O(6)–Co(1)–O(14) = 174.33(14)°]. Such arrangement of the coordinated oxygen atoms of the oxolinato ligands around Co(II) has been also observed in the crystal structure of [Ni(oxo)2(bipy)] complex (cis pyridone oxygen atoms [O(1)–Ni(1)–O(6) = 90.67(6)°] and trans carboxylato oxygens [O(2)–Ni(1)–O(7) = 173.02(6)°]),12 while in the crystal structure of the [Zn(oxo)2(phen)]·2MeOH complex, the two coordinated carboxylato oxygen atoms were located in cis arrangement [O(1)–Zn–O(1)′ = 105.3(2)°] with the two pyridone oxygen atoms lying on trans positions [O(3)–Zn–O(3)′ = 178.2(2)°] of the octahedron.7,22
The three co-crystallized MeOH molecules form a network of hydrogen bonds in which interestingly only one of the two cobalt-coordinated oxolinato ligands is involved (O(50)–H(50)⋯O(22)–H(22)⋯O(14)) and (O(28)–H(28)⋯O(12), Fig. 1(B)). Such interactions result in the increase of the Co–O bond lengths in comparison with the ligand which is not involved in hydrogen bonding [Co(1)–O(14) = 2.070(3) Å vs. Co(1)–O(6) = 2.032(3) Å and Co(1)–O(2) = 2.094(3) Å vs. Co(1)–O(3) = 2.079(3) Å].
The bipy ligand is planar with the cobalt atom lying in this plane. The Co–N bond distances [2.114(4) and 2.123(4) Å] and the N(20)–Co(1)–N(21) angle [=76.99(16)°] are within the range of reported values of other chelating polycyclic α-diimines.27,61–63
Antimicrobial activity of the complexes
The antimicrobial activities of Hoxo and its complexes 1–6 were evaluated by monitoring the growth of two Gram-negative (X. campestris, E. coli) and two Gram-positive (B. subtilis and S. aureus) bacterial strains in the presence of concentrations of the compounds ranging from 0 to 64 μg mL−1; the obtained half-minimum inhibitory concentration (IC50) and the minimum inhibitory concentration (MIC) values are presented in Table 2.| Compound | E. coli | X. campestris | B. subtilis | S. aureus | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | |
| Hoxo | 0.96 | 2 | 0.88 | 2 | 0.36 | 1 | >64 | >64 |
| [Co(oxo)2(MeOH)2], 1 | 0.97 | 2 | 0.98 | 2 | 0.46 | 1 | >64 | >64 |
| [Co(oxo)2(bipy)], 2 | 0.97 | 2 | 1.05 | 2 | 0.53 | 1 | >64 | >64 |
| [Co(oxo)2(bipyam)], 3 | 0.92 | 2 | 1.24 | 2 | 0.46 | 1 | >64 | >64 |
| [Co(oxo)2(phen)], 4 | 0.92 | 2 | 1.34 | 2 | 0.48 | 1 | >64 | >64 |
| [Co(oxo)2(py)2], 5 | 0.96 | 2 | 0.93 | 2 | 0.44 | 1 | >64 | >64 |
| [Co(oxo)2(4bzpy)2], 6 | 0.94 | 2 | 0.89 | 2 | 0.48 | 1 | >64 | >64 |
Oxolinic acid and its cobalt(II) complexes 1–6 present inhibitory action against all the microorganisms tested, especially against both Gram-negative bacteria and B. subtilis, with the MIC values being in the range 1–2 μg mL−1 and the IC50 values in the range 0.36–1.34 μg mL−1. The respective MIC and IC50 values against the Gram-positive strain of S. aureus tested were not accurately determined as they were higher than 64 μg mL−1. Higher concentrations in the cultures could not be achieved without altering significantly the growth medium of the assay due to the limitation posed by the relatively low solubility of all compounds in DMSO.
It is clear that the complexes are similarly active as free Hoxo against the microorganisms tested (Table 2). The compounds exhibit similar activity against both gram-(−) bacteria tested (MIC = 2 μg mL−1), while they do not seem active against the most resistant S. aureus strain (IC50 and MIC > 64 μg mL−1). Regarding the calculated IC50 values it is shown that all complexes exhibit similar IC50 values against E. coli (0.92–0.97 μg mL−1), against X. campestris the IC50 value of the free Hoxo was 0.88 μg mL−1 whereas those of the complexes were similar or higher, in particular the ones of compounds 3 and 4 were 1.24 and 1.34 μg mL−1, respectively. Furthermore, against B. subtilis the IC50 value of the free ligand was 0.36 μg mL−1 while all complexes exhibited higher values, with the highest being obtained by compound 2 (0.53 μg mL−1).
It is evident that the activity of the complexes is mainly dependent on the presence of the oxolinato ligands. The complexes seem to be less active than the corresponding Co(II)–enrofloxacinato complexes recently reported;39 indeed, enrofloxacin, as a second-generation quinolone, and its compounds are expected to be more active than those of the first-generation quinolone oxolinic acid.4,7 Furthermore, among the five factors that may influence the antimicrobial activity of a complex (chelate effect of ligands, nature of ligands, nuclearity, total charge, existence and nature of counterions),66 the nature of the ligands (presumably the quinolone oxolinic acid) and the chelate effect of the (oxolinato and nitrogen-donor) ligands seem to contribute most to the antimicrobial activity of the complexes. There is little differentiation on the activity that could be attributed to the nature of the co-ligands and there are no differences between the complexes in regard to the nuclearity (mononuclear complexes), total charge (neutral complexes) and existence of counterions (no counterions).67
In conclusion, the best inhibition is provided by all complexes 1–6 against B. subtilis (MIC = 1 μg mL−1), while S. aureus exhibits resistance on the activity of the compounds. Although, the antibacterial activity of the complexes is similar to that of free Hoxo, the obtained IC50 and MIC values are significantly low. Thus, the complexes might be considered promising for their potency as antibacterial agents.
Interaction of the complexes with CT DNA
The activity of the quinolone compounds as antibacterial agents is focused on the inhibition of DNA replication by targeting essential type II bacterial topoisomerases such as DNA gyrase and topoisomerase IV;1–3,5 therefore, the investigation of the interaction of quinolones and their complexes with DNA is of great significance. As known, metal complexes bind to double-stranded DNA via covalent or noncovalent interactions. Covalent binding occurs when the replacement of a labile ligand of the complex by a nitrogen base of DNA takes place. Noncovalent DNA-interactions with the metal complexes include intercalation inside the DNA helix (via π → π stacking interaction of the complex and DNA nucleobases), groove binding along major or minor groove of DNA helix (as a result of van der Waals interaction or hydrogen-bonding or hydrophobic bonding) and external/electrostatic interactions (Coulomb forces between metal complexes and the phosphate groups being on the surface of DNA are developed).7,68DNA-binding study with UV spectroscopy
In the UV spectra of complexes 1–6 (1 × 10−5 M), the intense absorption bands observed in the spectra of the complexes can be attributed to the intraligand transition of the coordinated groups of quinolone ligands.7,12–14,21–23 The existence and the possible mode of interaction between each complex and CT DNA may be revealed by the changes observed on the intraligand transition bands of complexes 1–6 upon addition of CT-DNA in diverse [complex]/[DNA] mixing ratios (r) values. In general, the changes observed in the UV spectra upon titration reveal the existence of interaction between each complex and CT DNA and may usually give information on the interaction mode; a hypochromism attributed to π → π stacking interactions appears in the case of an intercalative binding mode, a hyperchromism is observed in the case of external binding, while a red-shift (bathochromism) may be observed as evidence of the DNA-helix stabilization.69,70In the UV spectrum of 2 (Fig. 2), both bands at 325 nm (band I) and 337 nm (band II) exhibit in the presence of increasing amounts of CT DNA a slight hypochromism up to 4% (Table 4) and do not show any noteworthy shift. Similar behaviour (slight variation of the intensity and no shifts of the bands) was observed for all complexes (Table 3). The results derived from the UV spectroscopy suggest that the complexes can bind tightly to CT DNA although the exact mode of binding cannot be merely proposed by UV spectroscopic titration studies and more techniques should be employed.
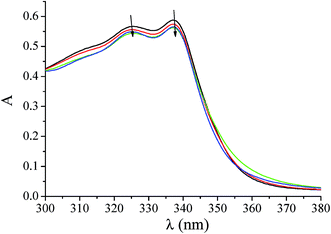 | ||
| Fig. 2 UV spectra of DMSO solution (1 × 10−5 M) of [Co(oxo)2(bipy)], 2 in the presence of increasing amounts of CT DNA. The arrows show the changes upon increasing amounts of CT DNA. | ||
| Compound | Band (λ, nm) (ΔA (%), Δλ (nm)) | Kb (M−1) |
|---|---|---|
| Hoxo22 | 3.02(±0.10) × 103 | |
| [Co(oxo)2(MeOH)2], 1 | 328 (−3.5, −3)/336 (−2.8, 0) | 1.80(±0.30) × 105 |
| [Co(oxo)2(bipy)], 2 | 325 (−3.0, +1)/337 (−3.9, 0) | 1.26(±0.20) × 105 |
| [Co(oxo)2(bipyam)], 3 | 325 (sh) (−1.5, 0)/337 (−2.5, 0) | 6.10(±0.10) × 105 |
| [Co(oxo)2(phen)], 4 | 325 (0, 0)/337 (−3.1, 0) | 3.03(±0.03) × 106 |
| [Co(oxo)2(py)2], 5 | 323 (−1.8, +1)/337 (0, 0) | 6.57(±0.33) × 105 |
| [Co(oxo)2(4bzpy)2], 6 | 326 (+2.5, −1)/337 (−1.3, 0) | 2.05(±0.25) × 105 |
The magnitude of the binding strength of complexes with CT DNA can be estimated through the binding constant Kb, which is calculated by the Wolfe–Shimer equation (eqn (S4)†)47 and plots 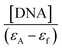 versus [DNA] (Fig. S1†). The Kb values calculated for complexes 1–6 (Table 3) are significantly higher than that of free oxolinic acid suggesting that its coordination to Co(II) results in a significant increase of the Kb value. The Kb values suggest a strong binding of the complexes to CT DNA, with complex 4 showing the highest Kb value (=3.03(±0.03) × 106 M−1) among the complexes, even higher than that of the classical intercalator EB (=1.23(±0.07) × 105 M−1) calculated in our lab.71 The Kb values of complexes 1–6 are of the same magnitude in comparison to other metal–quinolone complexes.7,12–14,21–23,64,65,68,72,73
versus [DNA] (Fig. S1†). The Kb values calculated for complexes 1–6 (Table 3) are significantly higher than that of free oxolinic acid suggesting that its coordination to Co(II) results in a significant increase of the Kb value. The Kb values suggest a strong binding of the complexes to CT DNA, with complex 4 showing the highest Kb value (=3.03(±0.03) × 106 M−1) among the complexes, even higher than that of the classical intercalator EB (=1.23(±0.07) × 105 M−1) calculated in our lab.71 The Kb values of complexes 1–6 are of the same magnitude in comparison to other metal–quinolone complexes.7,12–14,21–23,64,65,68,72,73
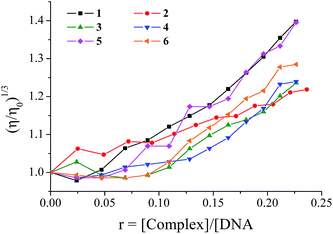
DNA-binding study with viscosity measurements
The measurement of the viscosity of a DNA solution upon addition of the complexes provides useful information in the attempt to their mode of interaction to DNA, since it is rather sensitive to DNA length changes due to the relation given by the equation L/L0 = (η/η0)1/3, with η/η0 denoting the relative solution viscosity and L/L0 the DNA length.68,72,73In the present case, the viscosity measurements were carried out on CT DNA solutions (0.1 mM) upon addition of increasing amounts of complexes 1–6 (up to the value of r = 0.24). The addition of the complexes to the DNA solution resulted in a significant increase of the relative viscosity of DNA (Fig. 3) which can be explained by the insertion of the complexes in between the DNA base pairs that leads to an increase in the separation of base pairs at intercalation sites and, thus, an increase in overall DNA length.63,68,72 The observed increase of the DNA viscosity in the presence of compounds may be considered an evidence of an intercalative binding mode to DNA; a conclusion which simply enforces the preliminary findings from UV spectroscopic studies showing a slight hypochromism which can now be considered evidence of intercalation.
Interaction with CT DNA monitored by cyclic voltammetry
The use of electrochemical techniques in the DNA–complexes interaction study provides useful information about the interaction of DNA with both the reduced and oxidized form of the complex. In the case of intercalation to DNA, the electrochemical potential will present a positive shift, and the shift of the potential to a negative direction occurs upon electrostatic interaction with DNA. When a positive shift of a potential (Ep1) occurs simultaneously with a negative shift of a second potential (Ep2), the molecule may bind to DNA by both intercalation and electrostatic interaction.74,75The cyclic voltammograms of each complex (0.4 mM) in 1/2 DMSO/buffer solution were studied upon addition of CT DNA (representatively in Fig. S2† for complexes 1 and 2) and the shifts of the cathodic Epc and anodic Epa potentials of the quasi-reversible redox couple Co(II)/Co(I) are given in Table 4. Upon addition of CT DNA to the complexes, no new redox peaks appeared and the current intensity exhibited a decrease which may be attributed to an equilibrium mixture of free and DNA-bound complex to the electrode surface suggesting, thus, the existence of interaction between each complex and CT DNA.74 For increasing amounts of CT DNA, the cathodic and the anodic potentials exhibited a positive shift (ΔEp = (+2) − (+23) mV) suggesting the existence of intercalation between the complexes and CT DNA bases;27,28,63,74 a conclusion being in accordance to spectroscopic and viscosity experiments.
| Complex | Epc(f)a | Epc(b)b | ΔEpcc | Epa(f)a | Epa(b)b | ΔEpac |
|---|---|---|---|---|---|---|
| a Epc/a in DMSO/buffer in the absence of CT DNA (Epc/a(f)).b Epc/a in DMSO/buffer in the presence of CT DNA (Epc/a(b)).c ΔEpc/a = Epc/a(b) − Epc/a(f). | ||||||
| [Co(oxo)2(MeOH)2], 1 | −739 | −716 | +23 | −580 | −570 | +10 |
| [Co(oxo)2(bipy)], 2 | −722 | −713 | +9 | −568 | −562 | +6 |
| [Co(oxo)2(bipyam)], 3 | −720 | −715 | +5 | −560 | −553 | +7 |
| [Co(oxo)2(phen)], 4 | −711 | −705 | +6 | −555 | −549 | +6 |
| [Co(oxo)2(py)2], 5 | −706 | −702 | +4 | −550 | −542 | +8 |
| [Co(oxo)2(4bzpy)2], 6 | −671 | −663 | +8 | −531 | −529 | +2 |
Competitive study with ethidium bromide
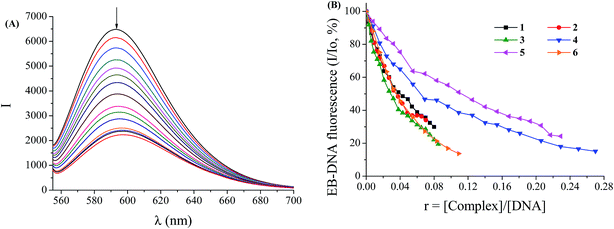
Ethidium bromide (EB = 3,8-diamino-5-ethyl-6-phenyl-phenanthridinium bromide) is an intercalator to CT DNA through the planar EB phenanthridine ring in between adjacent DNA base pairs. This intercalation results in the appearance of intense fluorescence emission band at 592 nm, when excited at 540 nm, due to the formation of the EB–DNA compound. Therefore, EB is a typical indicator of intercalation since a quenching of the DNA-induced EB fluorescence emission may appear when a compound which can intercalate to DNA equally or more strongly than EB, is added into a solution of the EB–DNA compound.76 Complexes 1–6 do not show any significant fluorescence at room temperature in solution or in the presence of CT DNA under the same experimental conditions and their addition to a solution containing EB does not provoke quenching of free EB fluorescence and new peaks do not appear in the spectra. Within this context, the changes observed in the fluorescence emission spectra of a solution containing the EB–DNA compound upon addition of the complexes can be used to investigate the ability of the complexes to displace EB from the EB–DNA complex.The fluorescence emission spectra of pre-treated EB–DNA ([EB] = 20 μM, [DNA] = 26 μM) were recorded for increasing amounts of the complexes up to the value of r = 0.27 (Fig. 4(A)). The addition of complexes 1–6 at diverse r values resulted in a significant decrease of the intensity of the emission band of the DNA–EB system at 592 nm (the final fluorescence is up to 14–34% of the initial EB–DNA fluorescence intensity in the presence of the complexes, Table 5) indicating the competition of the complexes with EB in binding to DNA (Fig. 4(B)). The observed quenching of DNA–EB fluorescence by the complexes suggests that the complexes can significantly displace EB from the DNA–EB compound, thus revealing the interaction with CT DNA by the intercalative mode.7,11–14,21–23,27,28,63–65,68,72–74
| Complex | ΔI/Io (%) | KSV (M−1) |
|---|---|---|
| [Co(oxo)2(MeOH)2], 1 | 70.0 | 2.98(±0.07) × 105 |
| [Co(oxo)2(bipy)], 2 | 66.0 | 3.09(±0.06) × 105 |
| [Co(oxo)2(bipyam)], 3 | 80.5 | 2.84(±0.05) × 105 |
| [Co(oxo)2(phen)], 4 | 84.5 | 3.95(±0.07) × 105 |
| [Co(oxo)2(py)2], 5 | 76.0 | 2.87(±0.07) × 105 |
| [Co(oxo)2(4bzpy)2], 6 | 86.0 | 1.39(±0.10) × 106 |
The Stern–Volmer plots of DNA–EB for the complexes (Fig. S3†) illustrate that the quenching of EB–DNA by the complexes was in good agreement (R = 0.99) with the linear Stern–Volmer equation (eqn (S2)†) proving that the replacement of EB from EB–DNA by each compound results in a decrease in the fluorescence intensity.27,28,68,74 The obtained values of KSV (Table 5) are relatively high showing the tight binding of the complexes to DNA with complex 4 exhibiting the highest Ksv value (=3.95(±0.07) × 105 M−1) among the complexes. The KSV values of complexes 1–6 are of the same magnitude to those of a series of metal complexes with quinolones as ligands.7,23,68
Interaction of complexes with serum albumins
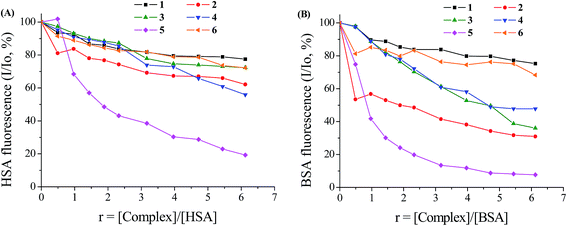
Serum albumin (SA) is among the most abundant proteins in plasma bearing the mission is to carry ions and drugs through the blood stream to cells and tissues. Therefore, the investigation of the interaction of potential drugs with SA is important as a means to discover differentiated biological properties of the drug or novel transport pathways.40 Within this context, the interaction of complexes 1–6 with human serum albumin (HSA) and its homologue bovine serum albumin (BSA) has been studied from tryptophan emission-quenching experiments. The solutions of HSA, bearing one tryptophan (Trp-214) and BSA (with two tryptophans, Trp-134 and Trp-212) exhibit an intense fluorescence emission when excited at 295 nm, with λem,max = 351 nm and 343 nm, respectively. The oxolinato complexes 1–6 exhibited an emission band with λem,max at 365 nm under the same experimental conditions; thus, the SA fluorescence spectra were corrected before the experimental data processing.7,23,68 The inner-filter effect was calculated by eqn (S3)† and slightly affected the measurements.50Addition of complexes 1–6 to a HSA solution results in relatively low to moderate fluorescence quenching, as calculated after the correction of the initial fluorescence spectra, for complexes 1–4 and 6 and significant (∼80%) for 5 (Fig. 5(A)). The quenching of BSA fluorescence upon addition of complexes 1–6 is much more pronounced up to ∼92% for complex 5 (Fig. 5(B)). This quenching could be attributed to possible changes in protein secondary structure of SA indicating the binding of the compounds to SA.49
The quenching constant values (kq) for the interaction of complexes 1–6 with the albumins have been calculated with Stern–Volmer quenching equation (eqn (S4)†) and the corresponding Stern–Volmer plots (Fig. S4 and S5†) and are given in Table S2† and are depicted in Fig. 6. The kq values (>1012 M−1 s−1) are higher than diverse kinds of quenchers for biopolymers fluorescence (2.0 × 1010 M−1 s−1) indicating the existence of static quenching mechanism.77 These values indicate good SA quenching ability and the kq values of the complexes are higher, in most cases, than the corresponding values of free Hoxo, with 5 exhibiting the highest quenching ability for both albumins (kq(HSA),5 = 2.18(±0.12) × 1013 M−1 s−1 and kq(BSA),5 = 7.09(±0.24) × 1013 M−1 s−1). The values of quenching constant are within the range found for a series of metal–quinolone complexes.7,23,68
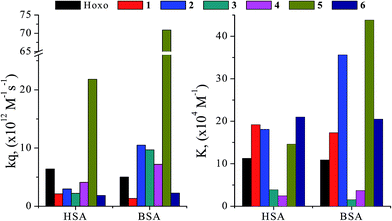 | ||
| Fig. 6 The BSA and HSA quenching constants (kq) and binding constants (K) derived for Hoxo and complexes 1–6. | ||
The values of the association binding constant (K) for the complexes as calculated from the Scatchard equation (eqn (S6)†) and the corresponding Scatchard plots (Fig. S6 and S7†) are given in Table S2† and Fig. 6. The relatively high K values of the complexes are, in most cases, higher than those of free Hoxo with 6 having the highest K value for HSA (K = 2.10(±0.10) × 105 M−1), while in the case of BSA complex 5 exhibits the highest K value among the compounds (K = 4.38(±0.19) × 105 M−1). Comparing the affinity of the complexes for BSA and HSA (K values), it is obvious that complexes 2, 4 and 5 show higher affinity for BSA than HSA with 3 showing higher binding constant for HSA, while the K values for BSA and HSA are similar for complexes 1 and 6. In general, the K values of the complexes are of the same magnitude with those calculated for a series of metal–quinolone complexes.7,23,68
In general, the values of the binding constants (K) of the complexes to SA are in the range 1.55 × 104 to 4.38 × 105 M−1 and could be considered high enough to suggest their binding to SAs and possible transfer and not too high, since they are quite below the value of the association constant (K ≈ 1015 M−1) of the strongest known non-covalent interactions between avidin and diverse ligands, so that they can get released from SAs upon arrival at the target cells.78
Molecular docking of complexes 1–6 into CT DNA and serum albumins
3D molecular models of complexes 1–6 are illustrated in Fig. S8† while the lower binding energy docking poses of the complexes in the crystal structure of CT DNA, HSA and BSA are depicted in Fig. S9–S11,† respectively. Binding energies of complexes 1–6 (in kcal mol−1) with CT DNA, HSA and BSA are shown in Table 6. From these data, it is deduced that the binding capacity of the complexes reduces (lower binding energy means higher binding capacity) in the order: 2 > 1 > 4 > 3 > 5 > 6. Similarly, the order of reducing binding capacity of the complexes bound to HSA and BSA is: 1 > 2 > 3 > 6 > 5 > 4 and 6 > 2 = 5 > 3 > 1 > 4, respectively. From these data, it is obvious that [Co(oxo)2(bipy)], 2, seems to succeed the best binding among complexes 1–6 for the DNA and both SAs. Our models for predicted binding poses of complexes into CT DNA suggest that all compounds are bound at the minor groove of DNA (Fig. S9†). Molecular docking pose of [Co(oxo)2(bipy)] (2) in the crystal structure of CT DNA is depicted in Fig. 7 where oxolinato and bipy moieties clearly show the binding of the molecule in the DNA minor-groove. Ligand binding site interactions of the complex in the binding pocket of minor-groove are illustrated in Fig. S12.† The nucleotides and the atoms of the complex involved in these binding interactions, along with bond lengths and type of interaction, are shown in Table S3.†| Compound | CT DNA | HSA | BSA |
|---|---|---|---|
| [Co(oxo)2(MeOH)2], 1 | −47.22 | −68.18 | −35.91 |
| [Co(oxo)2(bipy)], 2 | −51.31 | −58.65 | −46.69 |
| [Co(oxo)2(bipyam)], 3 | −38.36 | −48.01 | −42.73 |
| [Co(oxo)2(phen)], 4 | −46.35 | −44.37 | −33.82 |
| [Co(oxo)2(py)2], 5 | −37.90 | −45.47 | −46.60 |
| [Co(oxo)2(4bzpy)2], 6 | −12.40 | −47.44 | −54.62 |
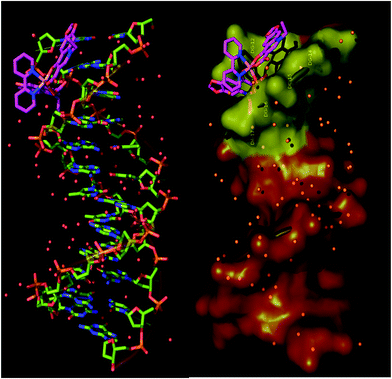 | ||
| Fig. 7 Molecular docking of [Co(oxo)2(bipy)] (2) in the crystal structure of CT DNA (PDB ID 1BNA). DNA structure is illustrated as cartoon color-coded according to chain (green C atoms) (left) or semitransparent surface in brown color with interacting nucleotides in the binding cavity of minor groove of DNA highlighted in yellow, while the superimposed docked molecule is represented in stick model and colored according to atom type (hot pink C atoms). Hydrogen atoms are omitted from all molecules for clarity. The final structure was ray-traced. | ||
The analysis of the ligand binding mode of [Co(oxo)2(bipy)], 2, inside the ligand-binding pockets of proteins HSA and BSA, along with the corresponding bond lengths between atoms of the oxolinato and bipy moieties and the amino-acid residues of the binding site, as long as the type of interaction, are shown in Tables S4 and S5.† Best docking poses of complexes 1–6 and the co-crystallized drug IBP in the crystal structure of HSA target protein (PDB ID 2BXG) are illustrated in Fig. S10.† More specifically, the simulated binding of complex 2 in HSA target protein is depicted in Fig. 8. Three drug-binding sites have been identified totally and are common with those of many other molecules crystallized with this protein. Three individual poses are depicted for the complex in domains IB, IIA and IIIB (at binding sites I, II and V, respectively). IBP is bound preferably at domains IIIA (where many ligands have been found to bind preferentially) and between IIA and IIB (binding sites IV and III, respectively). The results indicate that the primary binding site (with lower binding energy) is site II in the domain IIA, in a binding pocket formed between the subdomain helices: IIA-h1, IIA-h2, IIA-h3, IIA-h5 and IIA-h6. It is interesting that subdomains IIA and IIIA are the locations for the primary fatty-acid and bilirubin-binding sites. The other two binding sites (less preferable with higher binding energy) involve site I in domain IB inside the pocket formed by subdomain helices IB-h1, IB-h2, IB-h3 and IB-h4 (same place with biding of heme molecule) and site V in domain IIIB in a cavity formed by IIIB-h1, IIIB-h2, IIIB-h3 and IIIB-h4 helices. In a close-up view of the ligand binding pocket of HSA (Fig. 9), it is shown that complex 2 is bound in a cavity formed by the basic amino acid residues His288, Arg218, Arg22, Lys195 and Lys199 and additionally with the aromatic Tyr452 and Trp214. In Fig. S13† (ligand binding site interactions of complex 2 and co-crystallized drug IBP docked onto HSA), it is shown that 2 is docked very close to IBP.
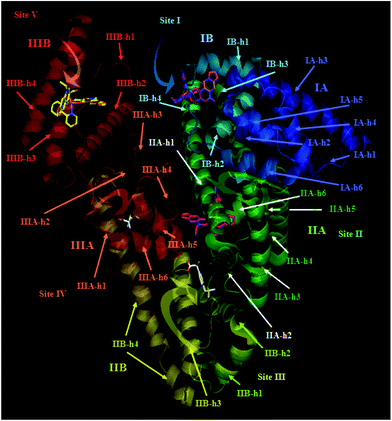 | ||
| Fig. 8 Docking poses of [Co(oxo)2(bipy)], 2 and the co-crystallized drug IBP into HSA (PDB ID 2BXG) (chain A). Target protein is illustrated as cartoon with sub-domains color-coded according to chainbow. Three individual poses are depicted for the complex and two for IBP in the most common binding sites: site I, II, III, IV and V in domains IA, IB, IIA, IIB, IIIA and IIIB. Complex 2 is docked in sites I, II and V (most favorable binding with lower binding energy in site II). Complex 2 and IBP molecules are depicted in stick model and colored according to atom type: violet-purple, hot pink and yellow carbon atoms for complex 2 bound in sites I, II and V, respectively, and white carbon atoms for IBP in binding sites III and IV. | ||
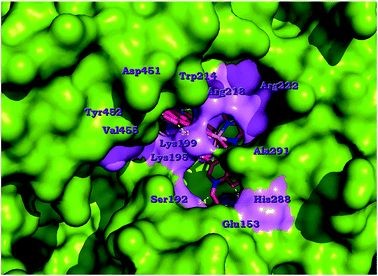 | ||
| Fig. 9 Ligand binding pocket of HSA target protein (PDB ID 2BXG) (chain A) with bound complex [Co(oxo)2(bipy)], 2. The protein is illustrated as surface colored by chain (in green) with additional depiction of selected contacting amino acid residues of the binding pocket highlighted in light magenta. Yellow dotted lines indicate hydrogen bond, polar and hydrophobic interactions between the docked molecule and the interacting amino acid residues. Hydrogen atoms are omitted for clarity. The final structure was ray-traced. | ||
Molecular docking of [Co(oxo)2(bipy)], 2 and the co-crystallized drug NPS in the crystal structure of BSA target protein (PDB ID 4OR0) is depicted in Fig. 10, while their interactions with the amino-acid residues of the binding pocket are shown in Fig. S14.† From these figures, it is obvious that complex 2 is practically anchored with one of its oxolinato moieties at the same place with NPS at binding site II which is a binding pocket formed between the subdomains IIA-h1, IIA-h2, IIA-h3, IIA-h5 and IIA-h6 (names of domains and subdomains are the same as for target protein HSA).
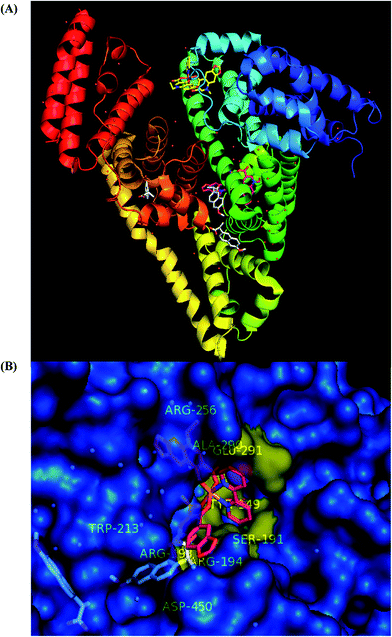 | ||
| Fig. 10 Molecular docking of [Co(oxo)2(bipy)], 2, and the co-crystallized drug NPS in the crystal structure of BSA target protein (PDB ID 4OR0) (chain A). (A) Target protein is illustrated as cartoon with sub-domains color-coded according to chainbow, while superimposed docked molecules are represented in stick model and colored according to atom type: complex 2 (yellow and hot pink C atoms for binding at sites I and II, respectively) and NPS (white C atoms for binding at sites II, III and IV). Binding sites are the same as for HSA. Dockings of both ligands were performed individually. Hydrogen atoms are omitted from all molecules for clarity. The final structure was ray-traced. (B) A close-up view of the ligand binding pocket of the protein illustrated as semitransparent surface colored by chain (in blue), with interacting amino acid residues in the binding cavity of the protein highlighted in yellow and complex 2, NPS molecules (rendered in stick model with hot pink and white C atoms, respectively) bound in site II. Hydrogen atoms are omitted for clarity. | ||
Conclusions
The synthesis and characterization of the mononuclear cobalt(II) complexes with the first-generation quinolone antibacterial agent oxolinic acid in the presence of the oxygen-donor methanol and the nitrogen-donor heterocyclic ligands 2,2′-bipyridine, 2,2′-bipyridylamine, 1,10-phenanthroline, pyridine or 4-benzyl-pyridine was achieved. In the resultant complexes, oxolinato ligands are deprotonated being bidentately coordinated to cobalt via the pyridone and a carboxylato oxygen. The crystal structure of the complex [Co(oxo)2(bipy)], 2, was determined by X-ray crystallography revealing a distorted octahedral geometry for Co(II) presenting a similar arrangement of the atoms around cobalt with previously reported Ni–oxolinato complexes.The antimicrobial activity of the complexes as estimated by the MIC values is comparable to that of free Hoxo against the bacteria tested. The best inhibition of complexes 1–6 is against B. subtilis, while the compounds seem to be inactive against the most resistant microorganism S. aureus in the range of the concentrations tested.
The interaction of all complexes with bovine or human serum albumins was studied by fluorescence emission spectroscopy revealing their tight binding affinity to BSA and HSA with relatively high binding constants (K = 1.55 × 104 to 4.38 × 105 M−1) which are in the optimum range to allow binding, transfer and release upon arrival at their targets.
UV spectroscopic studies, viscosity measurements and cyclic voltammetry revealed the ability of the complexes to bind to CT DNA. The complex [Co(oxo)2(phen)], 4, exhibits the highest binding strength to CT DNA, Kb (=3.03(±0.03) × 106 M−1), among the complexes examined, which is higher than the Kb value of EB calculated in our lab. Competitive binding studies with EB revealed that the complexes can displace significantly the typical intercalator EB from the EB–DNA complex indicating intercalation as the most possible interaction mode to CT DNA, a conclusion in accordance to the viscometry and cyclic voltammetry interaction studies.
The models for HSA and BSA complexation with complex 2 suggest that it could be bound at different sites of the serum proteins, which accommodates better their transportation. Due to different domain of both proteins taking place in the binding of complex 2, no common amino-acid residue was found for these interactions. Molecular modeling calculations can provide a molecular basis for the understanding of both the impairment of DNA by its binding with the studied complexes and also the ability of these compounds for transportation through blood serum albumin and possible interaction with other protein targets involved in various diseases.
The present study is the first report concerning metal–quinolone complexes where a combination of experimental data and in silico approaches with the employment of molecular docking simulations in regard the interaction with DNA and serum albumins in an attempt to provide information for the elucidation of the mechanism of action of the complexes (especially for complex 2) at a molecular level.
Abbreviations
| 4bzpy | 4-Benzylpyridine |
| B. subtilis | Bacillus subtilis |
| bipy | 2,2′-Bipyridine |
| bipyam | 2,2′-Bipyridylamine |
| BSA | Bovine serum albumin |
| CT | Calf-thymus |
| DMF | N,N-Dimethylformamide |
| E. coli | Escherichia coli |
| EB | Ethidium bromide = 3,8-diamino-5-ethyl-6-phenyl-phenanthridinium bromide |
| Hoxo | Oxolonic acid = 5,8-dihydro-5-ethyl-8-oxo-1,3-dioxolo[4,5-g]quinoline-7-carboxylic acid |
| HSA | Human serum albumin |
| IBP | Ibuprofen |
| IC50 | Half-minimum inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| NPS | Naproxen |
| oxo | Oxolinato anion |
| PDB | Protein Data Bank |
| phen | 1,10-Phenanthroline |
| py | Pyridine |
| r | [Compound]/[CT DNA] mixing ratio |
| rmsd | Root-mean squared deviation |
| s | Strong |
| S. aureus | Staphylococcus aureus |
| SA | Serum albumin |
| sh | Shoulder |
| vs | Very strong |
| X. campestris | Xanthomonas campestris |
| Δ | νasym(CO2) − νsym(CO2) |
Acknowledgements
This research has been co-financed by European Social Fund (ESF) and Greek national funds (National Strategic Reference Framework (NSRF)): Archimides III. This project was supported by the Slovenian Research Agency (ARRS) through project P1-0175 and partially by the infrastructure of the EN-FIST, Center of Excellence, Ljubljana, Slovenia. The project was also supported by EU COST Action CM1105.References
- The Quinolones, ed. V. T. Andriole, Academic Press, 2000 Search PubMed
.
- D. E. King, R. Malone and S. H. Lilley, Am. Fam. Physician, 2000, 61, 2741–2748 CAS
.
- I. Turel, Coord. Chem. Rev., 2002, 232, 27–47 CrossRef CAS
.
- V. Uivarosi, Molecules, 2013, 18, 11153–11197 CrossRef CAS PubMed
.
- N. Ahmed, M. Dawson, C. Smith and E. Wood, Biology of Disease, Taylor & Francis, 2007, ch. 3, pp. 41–71 Search PubMed
.
- J. Tuma, W. H. Connors, D. H. Stitelman and C. Richert, J. Am. Chem. Soc., 2002, 124, 4236–4246 CrossRef CAS PubMed
.
- G. Psomas and D. P. Kessissoglou, Dalton Trans., 2013, 42, 6252–6276 RSC
.
- N. Jimenez-Garrido, L. Perello, R. Ortiz, G. Alzuet, M. Gonzalez-Alvarez, E. Canton, M. Liu-Gonzalez, S. Garcia-Granda and M. Perez-Priede, J. Inorg. Biochem., 2005, 99, 677–689 CrossRef CAS PubMed
.
- P. Drevensek, T. Zupancic, B. Pihlar, R. Jerala, U. Kolitsch, A. Plaper and I. Turel, J. Inorg. Biochem., 2005, 99, 432–442 CrossRef CAS PubMed
.
- I. Turel, J. Kljun, F. Perdih, E. Morozova, V. Bakulev, N. Kasyanenko, J. A. W. Byl and N. Osheroff, Inorg. Chem., 2010, 49, 10750–10752 CrossRef CAS PubMed
.
- Y. Wang, R. Hu, D. Jiang, P. Zhang, Q. Lin and Y. Wang, J. Fluoresc., 2011, 21, 813–832 CrossRef CAS PubMed
.
- K. C. Skyrianou, F. Perdih, I. Turel, D. P. Kessissoglou and G. Psomas, J. Inorg. Biochem., 2010, 104, 161–170 CrossRef CAS PubMed
.
- K. C. Skyrianou, V. Psycharis, C. P. Raptopoulou, D. P. Kessissoglou and G. Psomas, J. Inorg. Biochem., 2011, 105, 63–74 CrossRef CAS PubMed
.
- A. Tarushi, C. P. Raptopoulou, V. Psycharis, A. Terzis, G. Psomas and D. P. Kessissoglou, Bioorg. Med. Chem., 2010, 18, 2678–2685 CrossRef CAS PubMed
.
- I. Turel, A. Golobic, A. Klavzar, B. Pihlar, P. Buglyo, E. Tolis, D. Rehder and K. Sepcic, J. Inorg. Biochem., 2003, 95, 199–207 CrossRef CAS
.
- M. P. Lopez-Gresa, R. Ortiz, L. Perello, J. Latorre, M. Liu-Gonzalez, S. Garcia-Granda, M. Perez-Priede and E. Canton, J. Inorg. Biochem., 2002, 92, 65–74 CrossRef CAS
.
- K. C. Skyrianou, E. K. Efthimiadou, V. Psycharis, A. Terzis, D. P. Kessissoglou and G. Psomas, J. Inorg. Biochem., 2009, 103, 1617–1625 CrossRef CAS PubMed
.
- E. K. Efthimiadou, H. Thomadaki, Y. Sanakis, C. P. Raptopoulou, N. Katsaros, A. Scorilas, A. Karaliota and G. Psomas, J. Inorg. Biochem., 2007, 101, 64–73 CrossRef CAS PubMed
.
- M. E. Katsarou, E. K. Efthimiadou, G. Psomas, A. Karaliota and D. Vourloumis, J. Med. Chem., 2008, 51, 470–478 CrossRef CAS PubMed
.
- D. J. D'Alessio, V. M. Olexy and G. G. Jackson, Antimicrob. Agents Chemother., 1967, 7, 490–496 Search PubMed
.
- G. Psomas, A. Tarushi, E. K. Efthimiadou, Y. Sanakis, C. P. Raptopoulou and N. Katsaros, J. Inorg. Biochem., 2006, 100, 1764–1773 CrossRef CAS PubMed
.
- A. Tarushi, G. Psomas, C. P. Raptopoulou and D. P. Kessissoglou, J. Inorg. Biochem., 2009, 103, 898–905 CrossRef CAS PubMed
.
- M. Zampakou, M. Akrivou, E. G. Andreadou, C. P. Raptopoulou, V. Psycharis, A. A. Pantazaki and G. Psomas, J. Inorg. Biochem., 2013, 121, 88–99 CrossRef CAS PubMed
.
- P. V. Bernhardt and G. A. Lawrance, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, 2003, vol. 6, ch. 1, pp. 1–45 Search PubMed
.
- P. J. Sadler, Adv. Inorg. Chem., 1991, 36, 1–48 CrossRef CAS
.
- M. D. Hall, T. W. Failes, N. Yamamoto and T. W. Hambley, Dalton Trans., 2007, 3983–3990 RSC
.
- F. Dimiza, A. N. Papadopoulos, V. Tangoulis, V. Psycharis, C. P. Raptopoulou, D. P. Kessissoglou and G. Psomas, Dalton Trans., 2010, 39, 4517–4528 RSC
.
- F. Dimiza, A. N. Papadopoulos, V. Tangoulis, V. Psycharis, C. P. Raptopoulou, D. P. Kessissoglou and G. Psomas, J. Inorg. Biochem., 2012, 107, 54–64 CrossRef CAS PubMed
.
- H. Lopez-Sandoval, M. E. Londono-Lemos, R. Garza-Velasco, I. Poblano-Melendez, P. Granada-Macias, I. Gracia-Mora and N. Barba-Behrens, J. Inorg. Biochem., 2008, 102, 1267–1276 CrossRef CAS PubMed
.
- I. Ott, A. Abraham, P. Schumacher, H. Shorafa, G. Gastl, R. Gust and B. Kircher, J. Inorg. Biochem., 2006, 100, 1903–1906 CrossRef CAS PubMed
.
- A. Bottcher, T. Takeuchi, K. I. Hardcastle, T. J. Meade and H. B. Gray, Inorg. Chem., 1997, 36, 2498–2504 CrossRef
.
- T. Takeuchi, A. Bottcher, C. M. Quezada, T. J. Meade and H. B. Gray, Bioorg. Med. Chem., 1999, 7, 815–819 CrossRef CAS
.
- D. U. Miodragovic, G. A. Bogdanovic, Z. M. Miodragovic, M. D. Radulovic, S. B. Novakovic, G. N. Kaludjerovic and H. Kozlowski, J. Inorg. Biochem., 2006, 100, 1568–1574 CrossRef CAS PubMed
.
- K. Nomiya, A. Yoshizawa, K. Tsukagoshi, N. C. Kasuga, S. Hirakawa and J. Watanabe, J. Inorg. Biochem., 2004, 98, 46–60 CrossRef CAS PubMed
.
- J. Lv, T. Liu, S. Cai, X. Wang, L. Liu and Y. Wang, J. Inorg. Biochem., 2006, 100, 1888–1896 CrossRef CAS PubMed
.
- Z. Weiqun, Y. Wen, X. Liqun and C. Xianchen, J. Inorg. Biochem., 2005, 99, 1314–1319 CrossRef PubMed
.
- M. P. Lopez-Gresa, R. Ortiz, L. Perello, J. Latorre, M. Liu-Gonzalez, S. Garcia-Granda, M. Perez-Priede and E. Canton, J. Inorg. Biochem., 2002, 92, 65–74 CrossRef CAS
.
- N. Jimenez-Garrido, L. Perello, R. Ortiz, G. Alzuet, M. Gonzalez-Alvarez, E. Canton, M. Liu-Gonzalez, S. Garcia-Granda and M. Perez-Priede, J. Inorg. Biochem., 2005, 99, 677–689 CrossRef CAS PubMed
.
- C. Protogeraki, E. G. Andreadou, F. Perdih, I. Turel, A. A. Pantazaki and G. Psomas, Eur. J. Med. Chem., 2014, 86, 189–201 CrossRef CAS PubMed
.
- J. He, D. Xiao, H. Chen, D. Sun, S. Yan, X. Wang, Z. Ye, Q. Luo and E. Wang, J. Solid State Chem., 2013, 198, 279–288 CrossRef CAS PubMed
.
- C. Tan, J. Liu, H. Li, W. Zheng, S. Shi, L. Chen and L. Ji, J. Inorg. Biochem., 2008, 102, 347–358 CrossRef CAS PubMed
.
- J. Marmur, J. Mol. Biol., 1961, 3, 208–211 CrossRef CAS
.
- M. F. Reichmann, S. A. Rice, C. A. Thomas and P. Doty, J. Am. Chem. Soc., 1954, 76, 3047–3053 CrossRef CAS
.
- A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori and R. Spagna, J. Appl. Crystallogr., 1999, 32, 115–119 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS
.
- A. Wolfe, G. Shimer and T. Meehan, Biochemistry, 1987, 26, 6392–6396 CrossRef CAS
.
- G. Zhao, H. Lin, S. Zhu, H. Sun and Y. Chen, J. Inorg. Biochem., 1998, 70, 219–226 CrossRef CAS
.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed
.
- L. Stella, A. L. Capodilupo and M. Bietti, Chem. Commun., 2008, 4744–4746 RSC
.
- Y. Wang, H. Zhang, G. Zhang, W. Tao and S. Tang, J. Lumin., 2007, 126, 211–218 CrossRef CAS PubMed
.
- J. M. Andrews, J. Antimicrob. Chemother., 2001, 48(S1), 5–16 CrossRef CAS PubMed
.
- J. Wang, H. Liu, J. Zhao, H. Gao, L. Zhou, Z. Liu, Y. Chen and P. Sui, Molecules, 2010, 15, 5807–5817 CrossRef CAS PubMed
.
- H. R. Drew, R. M. Wing, T. Takano, C. Broka, S. Tanaka, K. Itakura and R. E. Dickerson, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 2179–2183 CrossRef CAS
.
- J. Ghuman, P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri and S. Curry, J. Mol. Biol., 2005, 353, 38–52 CrossRef CAS PubMed
.
- A. Bujacz, K. Zielinski and B. Sekula, Proteins, 2014, 82, 2199–2208 CrossRef CAS PubMed
.
- SPARTAN ′10 v.1.1.0, Suite 370 Irvine, CA 92612, U.S.A., http://www.wavefun.com.
- RCSB Protein Data Bank, operated by the Research Collaboratory for Structural Bioinformatics, http://www.rcsb.org.
- W. L. DeLano, The PyMOL molecular graphics system, DeLano Scientific, San Carlos, CA, USA, 2002 Search PubMed
.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, Wiley, New Jersey, 6th edn, 2009 Search PubMed
.
- B. A. Frenz and J. A. Ibers, Inorg. Chem., 1972, 11, 1109–1116 CrossRef CAS
.
- A. Grirrane, A. Pastor, A. Ienco, C. Mealli and A. Galindo, J. Chem. Soc., Dalton Trans., 2002, 3771–3777 RSC
.
- S. Tsiliou, L.-A. Kefala, F. Perdih, I. Turel, D. P. Kessissoglou and G. Psomas, Eur. J. Med. Chem., 2012, 48, 132–142 CrossRef CAS PubMed
.
- K. C. Skyrianou, C. P. Raptopoulou, V. Psycharis, D. P. Kessissoglou and G. Psomas, Polyhedron, 2009, 28, 3265–3271 CrossRef CAS PubMed
.
- K. C. Skyrianou, F. Perdih, I. Turel, D. P. Kessissoglou and G. Psomas, J. Inorg. Biochem., 2010, 104, 740–749 CrossRef CAS PubMed
.
- A. D. Russell, in Disinfection, Sterilization and Preservation, ed. S. S. Block, Lippincott Williams & Wilkins, Philadelphia, 5th edn, 2001, pp. 31–56 Search PubMed
.
- M. Zampakou, S. Balala, F. Perdih, S. Kalogiannis, I. Turel and G. Psomas, RSC Adv., 2015, 5, 11861–11872 RSC
.
- J. Kljun, I. Bratsos, E. Alessio, G. Psomas, U. Repnik, M. Butinar, B. Turk and I. Turel, Inorg. Chem., 2013, 52, 9039–9052 CrossRef CAS PubMed
.
- E. C. Long and J. K. Barton, Acc. Chem. Res., 1990, 23, 271–273 CrossRef CAS
.
- G. Pratviel, J. Bernadou and B. Meunier, Adv. Inorg. Chem., 1998, 45, 251–262 CrossRef CAS
.
- A. Dimitrakopoulou, C. Dendrinou-Samara, A. A. Pantazaki, M. Alexiou, E. Nordlander and D. P. Kessissoglou, J. Inorg. Biochem., 2008, 102, 618–628 CrossRef CAS PubMed
.
- A. Tarushi, K. Lafazanis, J. Kljun, I. Turel, A. A. Pantazaki, G. Psomas and D. P. Kessissoglou, J. Inorg. Biochem., 2013, 121, 53–65 CrossRef CAS PubMed
.
- A. Tarushi, J. Kljun, I. Turel, A. A. Pantazaki, G. Psomas and D. P. Kessissoglou, New J. Chem., 2013, 37, 342–355 RSC
.
- G. Psomas, J. Inorg. Biochem., 2008, 102, 1798–1811 CrossRef CAS PubMed
.
- M. T. Carter, M. Rodriguez and A. J. Bard, J. Am. Chem. Soc., 1989, 111, 8901–8911 CrossRef CAS
.
- W. D. Wilson, L. Ratmeyer, M. Zhao, L. Strekowski and D. Boykin, Biochemistry, 1993, 32, 4098–4104 CrossRef CAS
.
- V. Rajendiran, R. Karthik, M. Palaniandavar, H. Stoeckli-Evans, V. S. Periasamy, M. A. Akbarsha, B. S. Srinag and H. Krishnamurthy, Inorg. Chem., 2007, 46, 8208–8221 CrossRef CAS PubMed
.
- O. H. Laitinen, V. P. Hytonen, H. R. Nordlund and M. S. Kulomaa, Cell. Mol. Life Sci., 2006, 63, 2992–3017 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1055618. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra05308k |
| This journal is © The Royal Society of Chemistry 2015 |