Ligand-free Cu-catalyzed odorless synthesis of unsymmetrical sulfides through cross-coupling reaction of aryl/benzyl/alkyl halides with an aryl boronic acid/S8 system as a thiolating agent in PEG†
Abstract
In this article, we have presented a novel, efficient and environmentally benign method for one-pot, one-step and odorless synthesis of a wide range of unsymmetrical sulfides from the reaction of aryl/benzyl/alkyl halides with aryl boronic acids in the presence of S8, NaOH and a catalytic amount of CuI in PEG200 as green solvent at 40–60 °C. The products were obtained in moderate to excellent yields. More importantly, this reaction is applicable for the gram-scale preparation of the desired sulfides.
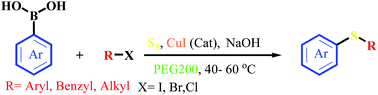

 Please wait while we load your content...
Please wait while we load your content...