Photoluminescence and photocatalytic activity of monodispersed colloidal “ligand free Ln3+-doped PbMoO4 nanocrystals”†
Abstract
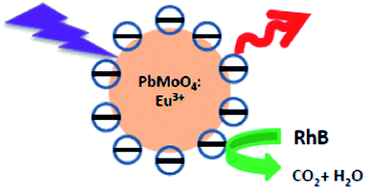
In this article, we have shown a synthetic strategy to prepare ligand-free colloidal Eu3+-doped PbMoO4 nanocrystals that form a stable dispersion in polar solvents. The surface of the nanocrystals is designed to have excess residual ions (Pb2+ or MoO42−) compared to the counter-ionic species, thereby providing a partially charged nanocrystal surface. These charged nanocrystal surfaces cause electrostatic repulsion, providing colloidal stability in polar solvents. Microscopic measurements suggest the formation of spherical shaped nanocrystals with an average size of 10 nm. Upon UV excitation, aqueous dispersions of the Eu3+-doped PbMoO4 nanocrystals display intense red emission characteristic of Eu3+ ions. Furthermore, under UV irradiation, the nanocrystals exhibit strong photocatalytic activity, which is verified from the degradation of rhodamine B dye. The rhodamine B dye is significantly degraded by ∼70% under UV illumination within 3 h at pH 5.5. The strong luminescence efficiency and photocatalytic activity make the water dispersible Ln3+-doped PbMoO4 nanocrystals a potential material for dual applications like luminescence and photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...