A 3D porous interconnected NaVPO4F/C network: preparation and performance for Na-ion batteries†
Abstract
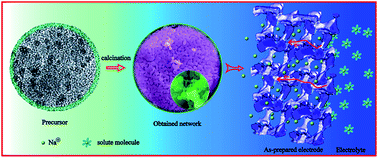
A uniform carbon embedded nano-scale NaVPO4F precursor of 3–5 nm is fabricated by a hydrothermal method and using vitamin C as the carbon source and reducing agent, followed by a sintering process, the carbon embedded NaVPO4F precursors transformed into a kind of 3D porous interconnected NaVPO4F/C network. As a Na-ion battery cathode material, the NaVPO4F/C network obtained at 750 °C achieves a high discharge capacity of 121 mA h g−1 and the sample obtained at 800 °C shows a slightly lower discharge capacity of 101 mA h g−1, but better rate capability and long cycle life. These results indicate that the 3D porous NaVPO4F/C network not only improves the electronic conductivity of the active material, but also prevents the aggregation of particles, and its open porous structure allows electrolyte penetration, and reduces the diffusion path of the sodium ions, hence maximizing utilization of the electrochemically active NaVPO4F particles.

 Please wait while we load your content...
Please wait while we load your content...