AMPK-mediated crosstalk of heteroglycan-induced reactive species and autophagic cascade in RAW 264.7 cells
Abstract
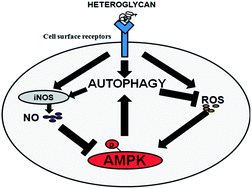
Natural polysaccharides are currently being employed for macrophage activation due to their minimal cytotoxicity and side effects. Reactive species like nitric oxide (NO) and reactive oxygen species (ROS), induced by a novel immunostimulatory heteroglycan were found to regulate the autophagic machinery in RAW 264.7 cells via a feedback mechanism, through AMPK. Heteroglycan-induced autophagy enhanced NO generation while itself being negatively regulated by NO. Concomitantly, intracellular ROS triggered the autophagic machinery which in turn subdued ROS levels indicating a homeostatic mechanism for regulated immune function. This study may help in better understanding of regulated immunostimulation by natural polysaccharides.

 Please wait while we load your content...
Please wait while we load your content...