DOI:
10.1039/C5RA05123A
(Paper)
RSC Adv., 2015,
5, 33705-33719
One-pot chemo/regio/stereoselective generation of a library of functionalized spiro-oxindoles/pyrrolizines/pyrrolidines from α-aroylidineketene dithioacetals†
Received
23rd March 2015
, Accepted 31st March 2015
First published on 31st March 2015
Abstract
An efficient chemo/regio/stereoselective synthesis of novel and functionalized spiro-oxindole/pyrrolizine/pyrrolidine scaffolds has been achieved. The in situ generated azomethine ylide from isatin & L-proline/phenyl alanine underwent 1,3-dipolar cycloaddition with α-aroylidineketene dithioacetals under simple reaction conditions affording spiro-oxindole derivatives. This protocol exhibits an interesting double bond selectivity of α-aroylidineketene dithioacetals. Furthermore, utilizing this spiro-oxindoles scaffold, biologically important benzimidazole and pyrimidine based poly heterocycles were also synthesized.
Introduction
Assembly of polycyclic frameworks is a fascinating topic of interest to many researchers in modern organic chemistry.1 The framework present in the spiro-oxindole core is part of a large number of bioactive, naturally occurring alkaloids and medicinally relevant compounds.2–4 For example, the naturally occurring (−)-horsfiline,5 spirotryptostatin A & B,6 (+)-elacomine,7 alstonisine8 and MI-129 (ref. 9) comprise spiro-oxindole skeleton (Fig. 1). These compounds were reported to show antibacterial, antitumor, antibiotic,10,11 antitubercular12 and anti-infective properties.13 At the same time, the highly substituted pyrrolidine system is constituted as core skeleton of many natural products.14
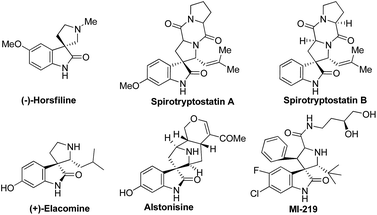 |
| | Fig. 1 Biologically important spiro-oxindoles/pyrrolidine scaffold. | |
The 1,3-dipolar cycloaddition is one of the most prominent protocol to construct spiro-oxindoles15 starting from simple substrates.16 This is exemplified by the increased number of publications depicting the synthesis of novel spiro heterocycles via 1,3-dipolar cycloaddition with different dipolarophiles in recent time.17–19 In particular, the multicomponent 1,3-dipolar cycloaddition of azomethine ylides generated in situ from the decarboxylative condensation of 1,2-dicarbonyl compounds and α-amino acids to exocyclic olefinic dipolarophiles have attracted a great deal of attention.20
In addition, cycloaddition on various dipolarophiles such as di and tribenzylidine acetone has been extensively studied.21 Recently, we have reported a chemo/regioselective synthesis of 6-pyrrolylpyrimidine by 1,3-dipolar cycloaddition of α-aroylidineketene dithioacetals, (p-tolylsulfonyl)methyl isocyanide (TosMIC) and guanidine nitrate via a multicomponent reaction.22
Multicomponent reactions23 (MCRs) are eco-friendly reactions wherein three or more components react to yield complex molecules with high atom economy by incorporating all the starting materials. MCRs are cost and time effective and afford the desired products in good yield under simple and mild reaction conditions24 in synthetic organic chemistry and drug discovery programs.25 These synthetic methods provide quick access to offer more powerful platform to assemble libraries of structurally complex molecules.26
Over the decades, α-aroylidineketene dithioacetals 3 have emerged as versatile intermediates in organic synthesis to synthesize substituted and fused aromatic heterocyclic frameworks.22,27 In the present work, we report the multicomponent reaction involving a 1,3-dipolar cycloaddition of 3, isatin and α-amino acid to afford spiro-oxindole derivatives. To the best of our knowledge, this is the first report on the synthesis of spiro-oxindole derivatives from α-aroyilidineketene dithioacetals 3.
Results and discussion
Following our reported procedure22 variety of α-aroylidineketene dithioacetals 3 were synthesized by condensing 4,4-bis(methylthio)but-3-en-2-one with various aryl/heteroaryl aldehydes under basic conditions in excellent yields. For the preliminary investigation, the cycloaddition of isatin 1a, L-proline 2a and 3l was performed in acetonitrile (ACN) at 70 °C for 90 min (Table 1, entry 1). This condition results only one product which was characterised as 2′-(3,3-bis(methylthio)acryloyl)-1′-(4-bromophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4l which reveals that the in situ generated azomethine ylide undergoes a 1,3-dipolar cycloaddition only on the double bond B of 3 rather than A. The more polar nature of push–pull alkene (A) of 3, prevents the double cycloaddition even though isatin and L-proline were taken as excess.
Table 1 Optimization of the reaction conditions for three component synthesis of 4la

|
| Entry |
Solvente |
Temp. (°C) |
Time (min) |
Yieldb (%) |
| α-Aroylidineketene dithioacetals 3l (1 mol), isatin 1a (1 mol) and L-proline 2a (1 mol), solvent (10 vol), temp., time. Isolated yields. Yields after column chromatography. Reaction time in hour. Reactions performed at the boiling points of the respective solvents. |
| 1 |
ACN |
70 |
90 |
90 |
| 2 |
EtOH |
90 |
90 |
89 |
| 3 |
MeOH |
65 |
60 |
99 |
| 4 |
MeOH |
rt |
120 |
65 |
| 5 |
MeOH/H2O |
65 |
90 |
65c |
| 6 |
EtOH/H2O |
80 |
90 |
70c |
| 7 |
IPA |
80 |
4d |
50 |
| 8 |
THF |
69 |
90 |
50c |
| 9 |
1,4-Dioxan |
90 |
60 |
78c |
| 10 |
DMF |
100 |
60 |
92c |
| 11 |
DCM |
40 |
90 |
96c |
| 12 |
Benzene |
65 |
60 |
88c |
| 13 |
Toluene |
100 |
60 |
85c |
This selective cycloaddition is significant to build a library of poly heterocycles for biological screening. An efficient way to generate 4l was encouraged us to further optimize the reaction conditions by varying the reaction temperature and solvent (Table 1).
Solvent plays a crucial role in the effective formation of 4l in high yield. Compound 4l was isolated in 89% yield, when isatin 1a and L-proline 2a was heated with dipolarophile 3l for 90 min at 90 °C in ethanol (Table 1, entry 2). Almost quantitative yield 99% was obtained when the cycloaddition was performed in methanol (Table 1 entry 3), while other alcoholic solvents such as ethanol, isopropyl alcohol (IPA) gave only moderate yields (Table 1 entries 1 & 7). Notably, cycloaddition using methanol at room temperature suppressed the product yield (Table 1, entry 4).
Meanwhile, diluting the reaction could dramatically reduce the yield of 4l (Table 1, entries 5 & 6). Aprotic solvents like THF (Table 1, entry 8) and 1,4-dioxane (Table 1, entry 9) provided reduced yields, while solvent like DMF (Table 1, entry 10) gave 4l in 92% yield. Quite impressively, the corresponding product 4l was obtained in 96% yield in dichloromethane (Table 1, entry 11). The product yield was drastically reduced, when the reaction was conducted in nonpolar solvents such as benzene and toluene (Table 1, entries 12 & 13).
Polar solvents afforded 4l as a solid after the initial work up without need of additional purification. The 1,3-dipolar cycloaddition of 3l (1 mol), isatin 1a (1 mol), and L-proline 2a (1 mol) in methanol at 65 °C for 60 min via MCR was found to be the best optimized reaction condition to afford 4l in excellent yield (Table 1, entry 3). We then examined the substrate scope and functional group tolerance of this new transformation under optimized condition, in order to find out the generality of the reaction (Table 2). A broad range of dipoles and dipolarophiles provided an access to diverse array of functionalized spiroindoline-pyrrolizine intermediates in excellent yield. Varying the substituents of dipolarophile, the reaction was compatible with all halogenated derivatives such as 4i, 4j, 4k, 4l, 4n, 4o and 4s in excellent yield (Table 2). Interestingly, dipolarophiles containing heterocyclic moieties such as 4t & 4u also provided the desired spiro scaffold without any difficulties (Table 2, entries 20 & 21).
Table 2 One-pot multicomponent synthesis of 2′-(3,3-bis(methylthio)acryloyl)-1′-(aryl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4a
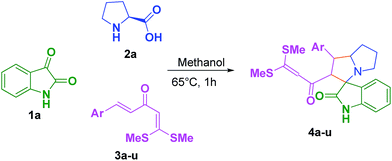
|
| Entry |
Ar 3 |
Product |
Yieldb (%) |
| Reaction conditions: 1a (1 mol), 2a (1 mol), 3a–u (1 mol), methanol (10 vol), reflux at 60 °C for 1 h. Isolated yield after recrystallization from ethanol–DCM mixture. |
| 1 |
C6H5 |
4a |
91 |
| 2 |
2-CH3–C6H4− |
4b |
94 |
| 3 |
4-CH3–C6H4− |
4c |
92 |
| 4 |
4-CH(CH3)2–C6H4 |
4d |
95 |
| 5 |
4-OEt–C6H4 |
4e |
96 |
| 6 |
2-OMe–C6H4 |
4f |
92 |
| 7 |
3-OMe–C6H4 |
4g |
90 |
| 8 |
4-OMe–C6H4 |
4h |
96 |
| 9 |
2-F–C6H4 |
4i |
93 |
| 10 |
4-F–C6H4 |
4j |
98 |
| 11 |
2-Br–C6H4 |
4k |
94 |
| 12 |
4-Br–C6H4 |
4l |
99 |
| 13 |
4-CN–C6H4 |
4m |
88 |
| 14 |
2,4-Cl2–C6H3 |
4n |
90 |
| 15 |
2,4-F2–C6H3 |
4o |
91 |
| 16 |
3,4-OMe–C6H3 |
4p |
90 |
| 17 |
2-OMe,5-Br–C6H3 |
4q |
86 |
| 18 |
2-Cl,5-NO2–C6H3 |
4r |
88 |
| 19 |
3-Br,4-F–C6H3 |
4s |
90 |
| 20 |
5-Br–C4H2S |
4t |
93 |
| 21 |
5-Br–C5H3N |
4u |
92 |
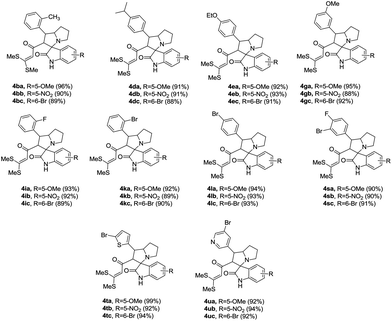
We explored the possibility of generating azomethine ylide from substituted isatin and L-proline. Derivatives of isatins 1b–d such as 5-methoxy, 5-nitro and 6-bromo were reacted with L-proline with various dipolarophiles underwent multicomponent cycloaddition to afford spiro-oxindoles 4 in excellent yields (Table 3). In all the cases, the product was isolated without the need of any additional purification (Table 3). The products 4 were well characterized by 1H, 13C, and mass spectral data.
Table 3 Scope of the isatina

|
| All the reactions were carried out with 1b–d (1 mol), 2a (1 mol) and 3 (1 mol) in 5 mL of MeOH and yields were isolated after recrystallization from ethanol–DCM mixture. |
 |
In order to determine the stereoselectivity, the structure of 4l (Fig. 2), 4db (Fig. 3) and 4kb (Fig. 4) was confirmed by single crystal X-ray analysis.28 This cycloaddition is stereoselective affording only one diastereomer of 4 exclusively, even though four stereocenters are present in these cycloadducts.
 |
| | Fig. 2 X-Ray crystal structures of 4l. | |
 |
| | Fig. 3 X-Ray crystal structures of 4db. | |
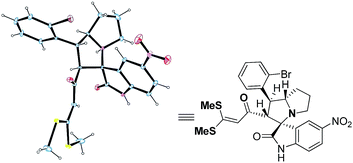 |
| | Fig. 4 X-Ray crystal structures of 4kb. | |
The present protocol was examined with 3l by performed on a larger scale to give 4l in excellent yield which reflects the generality and ease of performing on milligram scale (Scheme 1). It is worth mentioned that this could be highly important in the synthetic application.
 |
| | Scheme 1 Reaction of 1a, 2a and 3l in gram scale. | |
As shown in Scheme 2, we postulated the plausible mechanism for the regio and stereoselective formation of spiro-oxindoles. Initially, the reaction proceeds via condensation of 1 and 2 to furnish intermediate imine I followed by loss of CO2 to generate azomethine ylide II. Finally, electron rich carbon of azomethine ylide II undergoes 1,3-dipolar cycloaddition selectively with β-carbon of double bond B of 3 to afford spiro-cycloadducts 4 with four stereogenic center.
 |
| | Scheme 2 Plausible mechanism for 4. | |
To elaborate the scope of this present protocol, L-proline was replaced with primary amino acid such as L-phenyl alanine 2b (Scheme 3). The cycloaddition of 3b, 1a and L-phenylalanine 2b were performed under the optimized reaction conditions to furnish 60% yield of expected product 5b along with 2-((1Z,4E)-5-(4-methoxyphenyl)-1-(methylthio)-3-oxopenta-1,4-dienylamino)-2-phenylacetic acid 6 in 20% yield (Scheme 3, confirmed by LC-MS).
 |
| | Scheme 3 Three component synthesis of 5b using primary amino acid. | |
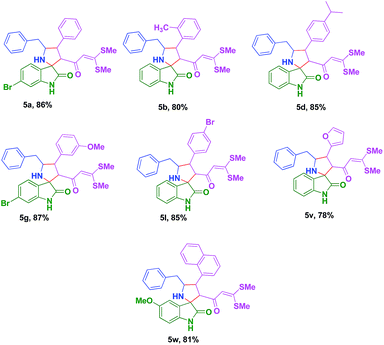
To avoid the formation of S,N-acetal 6, the sequential addition was performed by mixing phenylalanine 2b, isatin 1a together for 15 min, once the formation of azomethine ylide was confirmed the compound 3b was added to the mixture to reflux for 1 h. The sequential cycloaddition was successful to afford 5b in 80% yield. Using this technique several novel spiro-pyrrolidine 5 were synthesized in good yield (Table 4). The structure of 5g was confirmed on the basis of single crystal X-ray analysis28 (Fig. 5).
Table 4 Synthesis of functionalized spiro-pyrrolidine derivatives 5 via sequential additiona

|
| Reaction conditions: (i) 1 (1 mol), 2b (1 mol), methanol (10 vol), RT, 15 min. (ii) 3 (1 mol) reflux at 60 °C for 1 h. Yields after column chromatography. |
 |
 |
| | Fig. 5 X-Ray crystal structures of 5g. | |
Recently, aryl substituted ketene dithioacetals 3 has been reported to aid the synthesis of molecules which demonstrates antileishmanial activity.29 With the ready accessibility of spiro based diverse ketene dithioacetal scaffold established, next, the synthetic potential of 4 has been illustrated through the synthesis of new compounds with additional heterocycles such as benzimidazole and pyrimidine.
Poly heterocylces 8a was achieved from the cyclocondensation reaction30 of the 4h with OPD 7 in AcOH (cat.)/H2O media (Scheme 4). Inspired by the successful results, few spiro compounds 4 was investigated with OPD 7 under acidic condition. To optimize the best reaction condition, three different methods were executed to obtain 8. In method A, compound 4h and 7 was allowed to undergo cyclocondensation in dilute acid condition at 100 °C for 1 h to afford 8 in 78% yield. Water being a greened solvent was the preferred choice though similar results were observed with methanol and ethanol (Scheme 4, method A).
 |
| | Scheme 4 Utility of 4h towards the synthesis of spiro-benzimidazole 8a (method A). Reaction conditions: (i) 4h (1 mol), 7 (1 mol), AcOH/water, reflux, 1 h. | |
The cyclocondensation product 8a was confirmed by 1H & 13C NMR spectroscopy. Next, the cycloaddition was proceeding via MCR by involving 3h, 1a, 2a and OPD 7 in the presence of catalytic amount of AcOH in water at reflux for 1 h to afford 8a in 30% yield (Scheme 5 method B).
 |
| | Scheme 5 Synthesis of 8a via MCR (method B). Reaction conditions: (i) 3h (1 mol), 1a (1 mol), 2a (1 mol), and 7 (1 mol), AcOH (cat.)/H2O, reflux, 1 h. | |
We have acquired a new method to synthesize 8a even though MCR reaction gives 30% yield, as it involves additional purifications steps. Alternatively, in step-I, styryl benzimidazole 9 was synthesized in 87% yield by condensing 3h and 7 under AcOH/H2O media. In the step-II, styryl benzimidazole 9 was reacted with azomethine ylide (adduct of 1a and 2a) to give 8a in very poor yield (10%, Scheme 6).
 |
| | Scheme 6 Synthesis of spiro benzimidazole 8a via 2-styryl benzimidazole 9. | |
Among three different methods, method A (cyclization reaction of 4h with OPD 7) was the best on the basis of yields and isolation procedure. Following method A, biologically active poly heterocycles 8a–c were synthesised from corresponding substituted reagent 4 (Table 5). However, biologically important benzimidazole with spiro-oxindole skeleton is rare combinations in organic synthesis.
Table 5 Synthesis of poly heterocycles 8 containing benzimidazole moietya
| Reaction conditions: (i) 4h (1 mol), 7 (1 mol), AcOH/water, reflux, 1 h, yields given after column chromatography. |
 |
The curiosity towards the synthesis of poly heterocycles which contains biologically active pyrimidine moiety 10 was intensified by exploring our reported procedure.22 However, compound 4 was allowed to react with guanidine nitrate in the presence of NaH base under reflux condition for 10 h to afford 10a–d in good yields (Table 6).
Table 6 Synthesis of spiro-pyrimidines 10a–da

|
| Reaction conditions: (i) 4 (1 mol), guanidine nitrate (1.5 mol), NaH (1.5 mol), MeOH or EtOH, 65–70 °C, 10 h. Isolated yield after column chromatography. |
 |
The current work depicts the potential of α-aroylidineketene dithioacetal as key synthetic intermediates. The methodology described in this paper provides environmentally attractive synthetic approach with very high yields and atom efficiency. Moreover, this synthetic protocol provided a practical access to various biologically important N-based poly heterocycles with minimum number of synthetic steps.
Conclusions
In summary, we have demonstrated the straight forward chemo/regio/stereoselective synthesis of spiro-oxindole/pyrrolidine/derivatives in excellent yields with four chiral centers. The current method discloses many advantages such as high atom economy, ready accessibility of the starting materials, simple reaction conditions under greener medium. This protocol involves in broad substrate scope, excellent functional group tolerance and leaves active site for further synthetic transformation. Furthermore, the utility of cycloadduct was demonstrated through the synthesis of N-based poly heterocycles such as benzimidazole/pyrimidine moieties with spiro platform.
Experimental section
General remarks
Melting points were determined in open capillary tubes and were uncorrected. IR spectra were taken on a Jasco FT-IR instrument in KBr pellets and reported in cm−1. Mass spectra were performed with Agilent mass spectrometer and recorded in positive & negative mode with an ESI source. The 1H and 13C NMR spectra of the new compounds were measured at 300 and 400 MHz in DMSO-d6 with TMS as the internal standard. Chemical shifts are expressed in ppm, coupling constant (J values) are given in Hertz (Hz) and spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublets), td (triplet of doublets). Elemental analyses were carried out with Perkin Elmer 2400 Series II analyzer. Silica gel-G plates (Merck) were used for TLC analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as eluent. All chemicals were purchased and used without further purification.
General procedure for the synthesis of 4
A mixture of isatin 1 (1 mol), L-proline 2 (1 mol) and 1,1-bis(methylthio)-5-arylpenta-1,4-dien-3-one 3 (1 mol) was heated to reflux in methanol (3 ml) at 65 °C for indicated time (Table 2). After completion of the reaction (TLC), the mixture was cooled to room temperature and poured in to ice cold water. Then the resulting solid was filtered and recrystallized from ethanol–DCM to give analytically pure product 4.
2′-(3,3-Bis(methylthio)acryloyl)-1′-phenyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4a. Off white solid; isolated yield 0.328 g (91%); M. Pt. 160–162 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.69 (m, 2H), 1.78–1.83 (m, 2H), 2.15 (s, 3H), 2.23 (s, 3H), 2.33–2.37 (m, 1H), 2.40–2.49 (m, 1H), 3.68–3.77 (m, 2H), 4.00 (d, J = 11.9 Hz, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.92 (td, J = 0.4 Hz, 7.6 Hz, 1H), 7.14–7.26 (m, 3H), 7.33 (t, J = 7.6 Hz, 2H), 7.39 (d, J = 6.8 Hz, 2H), 10.46 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 15.5, 16.6, 27.4, 30.5, 47.6, 51.5, 67.9, 72.9, 73.5, 110.0, 112.1, 121.5, 126.1, 127.0, 127.2, 128.2, 129.0, 129.5, 141.2, 142.6, 163.4, 180.1, 189.0; IR (ATR KBr, cm−1) 702, 748, 1134, 1489, 1502, 1616, 1708, 2850, 3082, 3188; LC-MS calcd m/z: 450 found 451 [(M + 1)]+. Anal. calcd for C25H26N2O2S2: C, 66.63; H, 5.82; N, 6.22; found: C, 66.60; H, 5.77; N, 6.20.
2′-(3,3-Bis(methylthio)acryloyl)-1′-o-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4b. Off white solid; isolated yield 0.33 g (94%); M. Pt. 158–160 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.59–1.68 (m, 2H), 1.77–1.83 (m, 2H), 2.14 (s, 3H), 2.22 (s, 3H), 2.35–2.48 (m, 2H), 2.49 (s, 3H), 3.74–3.78 (m, 1H), 3.92–3.94 (m, 1H), 3.96 (d, J = 9.24 Hz, 1H), 5.52 (s, 1H), 6.77–6.79 (m, 1H), 6.92–6.96 (m, 1H), 6.98–7.09 (m, 1H), 7.14–7.25 (m, 4H), 7.38 (d, J = 7.32 Hz, 1H), 10.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.8, 17.2, 19.0, 19.8, 20.3, 27.6, 30.7, 46.3, 69.7, 73.5, 110.1, 111.8, 121.7, 126.1, 126.4, 126.5, 126.6, 126.7, 126.8, 127.0, 129.4, 129.5, 130.6, 131.2, 137.1, 137.6, 139.6, 142.5, 163.3, 180.1, 188.9; IR (ATR KBr cell, cm−1) 726, 1030, 1480, 1587, 1602, 1740, 2984, 3178; LC-MS calcd m/z: 464 found 465 [(M + 1)]+. Anal. calcd for C26H28N2O2S2: C, 67.21; H, 6.07; N, 6.03; found: C, 67.15; H, 6.03; N, 6.01.
2′-(3,3-Bis(methylthio)acryloyl)-1′-p-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4c. Off white solid; isolated yield 0.323 g (92%); M. Pt. 158–160 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.62–1.70 (m, 2H), 1.75–1.82 (m, 2H), 2.15 (s, 3H), 2.25 (s, 3H), 2.31 (s, 3H), 2.32–2.38 (m, 1H), 2.40–2.44 (m, 1H), 3.63–3.74 (m, 2H), 3.96 (d, J = 11.6 Hz, 1H), 5.62 (s, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.91 (t, J = 7.2 Hz, 1H), 7.11–7.17 (m, 3H), 7.27 (t, J = 8 Hz, 3H), 10.45 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 19.0, 21.0, 27.5, 30.5, 47.6, 51.2, 56.5, 67.9, 72.9, 73.4, 110.0, 112.1, 121.5, 126.1, 127.2, 128.0, 129.4, 129.5, 136.0, 138.1, 142.5, 163.3, 180.2, 189.0; IR (ATR KBr cell, cm−1) 730, 1148, 1430, 1500, 1616, 1726, 2800, 3298; LC-MS calcd m/z: 464 found 465 [(M + 1)]+. Anal. calcd for C26H28N2O2S2: C, 67.21; H, 6.07; N, 6.03: found; C, 67.18; H, 6.05; N, 6.00.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-isopropylphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4d. Off white solid; isolated yield 0.32 (95%); M. Pt. 134–136 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.17 (s, 3H), 1.19 (s, 3H), 1.62–1.68 (m, 2H), 1.76–1.81 (m, 2H), 2.14 (s, 3H), 2.32 (s, 3H), 2.33–2.38 (m, 1H), 2.40–2.44 (m, 1H), 2.80–2.87 (m, 1H), 3.43–3.47 (m, 1H), 3.63–3.76 (m, 2H), 3.98 (d, J = 11.6 Hz, 1H), 5.61 (s, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.91 (t, J = 7.6 Hz, 1H), 7.13–7.20 (m, 3H), 7.24 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 8 Hz, 2H), 10.46 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 19.0, 24.4, 27.5, 30.5, 47.6, 51.2, 56.5, 67.9, 72.9, 73.5, 110.0, 112.1, 121.5, 126.2, 127.2, 128.1, 129.4, 138.5, 142.6, 147.0, 163.3, 180.2, 189.0; IR (ATR KBr cell, cm−1) 750, 1136, 1483, 1616, 1728, 2980, 3217; LC-MS calcd m/z: 492 found 493 [(M + 1)]+. Anal. calcd for C28H32N2O2S2: C, 68.26; H, 6.55; N, 5.69; found: C, 68.22; H, 6.51; N, 5.65.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-ethoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4e. Off white solid; isolated yield 0.309 g (96%); M. Pt. 166–168 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.30 (t, J = 6.8 Hz, 1H), 1.62–1.68 (m, 2H), 1.75–1.82 (m, 2H), 2.15 (s, 3H), 2.24 (s, 3H), 2.31–2.36 (m, 1H), 2.38–2.44 (m, 1H), 3.61–3.72 (m, 2H), 3.92 (d, J = 11.6 Hz, 1H), 3.99 (q, J = 6.8 Hz, 2H), 5.62 (s, 1H), 6.76 (d, J = 7.6 Hz, 2H), 6.85–6.95 (m, 3H), 7.16 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 1H), 7.28 (d, J = 8.4 Hz, 2H), 10.44 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 15.2, 16.7, 27.4, 30.5, 47.6, 50.7, 63.4, 68.0, 72.9, 73.4, 110.0, 112.2, 114.9, 121.5, 126.2, 127.2, 129.1, 129.4, 132.8, 142.5, 157.7, 163.2, 180.2, 189.9.; IR (ATR KBr cell, cm−1) 713, 1192, 1571, 1640, 1716, 2870, 3280; LC-MS calcd m/z: 494 found 495 [(M + 1)]+. Anal. calcd for C27H30N2O3S2: C, 65.56; H, 6.11; N, 5.66; found: C, 65.53; H, 6.08; N, 5.64.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4f. Off white solid; isolated yield 0.315 g (92%); M. Pt. 138–140 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.70 (m, 2H), 1.76–1.81 (m, 3H), 2.14 (s, 3H), 2.22 (s, 3H), 2.31–2.40 (m, 2H), 3.64–3.69 (m, 1H), 3.82 (s, 3H), 4.03–4.08 (m, 1H), 4.24 (d, J = 12.4 Hz, 1H), 5.66 (s, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.94 (q, J = 7.2 Hz, 2H), 6.99 (d, J = 8 Hz, 1H), 7.14–7.21 (m, 3H), 7.32 (d, J = 6.4 Hz, 1H), 10.44 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.6, 31.1, 45.3, 47.3, 56.1, 66.0, 72.1, 73.4, 110.1, 111.7, 111.8, 121.1, 121.5, 126.4, 126.8, 128.0, 128.6, 129.4, 142.7, 158.0, 163.3, 180.0, 189.3; IR (ATR KBr cell, cm−1) 748, 1024, 1240, 1492, 1618, 1716, 2872, 2960, 3134; LC-MS calcd m/z: 480 found 481 [(M + 1)]+. Anal. calcd for C26H28N2O3S2: C, 64.97; H, 5.87; N, 5.83; found: C, 64.92; H, 5.83; N, 5.80.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4g. Off white solid; isolated yield 0.309 g (90%); M. Pt. 146–148 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.69 (m, 2H), 1.77–1.82 (m, 3H), 2.15 (s, 3H), 2.23 (s, 3H), 2.32–2.34 (m, 1H), 2.40–2.48 (m, 1H), 3.65–3.95 (m, 5H), 3.97 (d, J = 11.5 Hz, 1H), 5.63 (s, 1H), 6.75–6.79 (m, 2H), 6.91 (td, J = 0.76 Hz, 7.56 Hz, 1H), 6.95–6.97 (m, 2H), 7.17 (td, J = 0.96 Hz, 8 Hz, 1H), 7.21–7.26 (m, 2H), 10.48 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 19.0, 27.4, 30.4, 47.6, 51.5, 55.4, 56.5, 67.7, 72.8, 73.5, 110.0, 112.1, 112.2, 114.2, 120.2, 121.5, 126.1, 127.2, 129.5, 130.0, 142.6, 142.9, 159.8, 163.4, 180.1, 189.0; IR (ATR KBr cell, cm−1) 715, 781, 1051, 1268, 1379, 1480, 1649, 1729, 2873, 1046, 3189; LC-MS calcd m/z: 480 found 481 [(M + 1)]+. Anal. calcd for C26H28N2O3S2: C, 64.97; H, 5.87; N, 5.83; found: C, 64.93; H, 5.80; N, 5.78.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4h. Off white solid; isolated yield 0.329 g (96%); M. Pt. 154–156 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.68 (m, 2H), 1.75–1.81 (m, 2H), 2.15 (s, 3H), 2.24 (s, 3H), 2.30–2.34 (m, 1H), 2.35–2.41 (m, 1H), 3.60–3.72 (m, 5H), 3.91 (d, J = 11.56 Hz, 1H, 3H), 5.61 (s, 1H), 6.76 (d, J = 7.64 Hz, 1H), 6.87–6.92 (m, 3H), 7.16 (dd, J = 0.68 Hz, 7.64 Hz, 1H), 7.24 (d, J = 7.36 Hz, 1H), 7.30 (d, J = 7.36 Hz, 1H), 10.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 27.4, 30.5, 47.6, 50.7, 55.5, 68.0, 72.9, 73.4, 110.0, 112.1, 114.4, 121.5, 126.1, 127.2, 129.1, 129.4, 132.9, 142.5, 158.4, 163.2, 180.1, 189.0; IR (ATR KBr cell, cm−1) 750, 1041, 1234, 1479, 1604, 1728, 2892, 3064, 3199; LC-MS calcd m/z: 480 found 481 [(M + 1)]+. Anal. calcd for C26H28N2O3S2: C, 64.97; H, 5.87; N, 5.83; found: C, 64.93; H, 5.83; N, 5.79.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4i. Off white solid; isolated yield 0.325 g (93%); M. Pt. 160–162 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.68 (m, 2H), 1.79–1.84 (m, 2H), 2.15 (s, 3H), 2.26 (s, 3H), 2.32–2.48 (m, 2H), 3.73–3.78 (m, 1H), 3.90–3.95 (m, 1H), 4.17 (d, J = 12 Hz, 1H), 5.61 (s, 1H), 6.78 (d, J = 7.5 Hz, 1H), 6.94 (dd, J = 0.8 Hz, 7.2 Hz, 1H), 7.14–7.19 (m, 4H), 7.23–7.29 (m, 5H), 7.47–7.51 (m, 1H), 10.56 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.5, 30.7, 45.0, 47.4, 66.6, 71.7, 73.3, 110.1, 111.6, 115.8, 116.0, 121.6, 125.1, 125.9, 127.0, 127.6, 128.7, 128.8, 129.6, 129.7, 142.5, 159.9, 162.4, 163.7, 179.9, 188.5; IR (ATR KBr cell, cm−1) 729, 1390, 1488, 1640, 1720, 2850, 3184; LC-MS calcd m/z: 468 found 469 [(M + 1)]+. Anal. calcd for C25H25FN2O2S2: C, 64.08; H, 5.38; N, 5.98; found: C, 64.04; H, 5.33; N, 5.93.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4j. Off white solid; isolated yield 0.342 g (98%); M. Pt. 146–148 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.70 (m, 2H), 1.78–1.82 (m, 2H), 2.15 (s, 3H), 2.24 (s, 3H), 2.31–2.36 (m, 1H), 2.38–2.43 (m, 1H), 3.71–3.73 (m, 2H), 3.94–3.96 (m, 1H), 5.60 (s, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.91 (td, J = 0.68 Hz, 7.52 Hz, 1H), 7.12–7.18 (m, 3H), 7.24 (d, J = 7.4 Hz, 1H), 7.41–7.44 (m, 2H), 10.56 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.4, 30.3, 47.6, 50.6, 68.0, 72.7, 73.4, 110.0, 112.0, 115.5, 115.8, 121.5, 126.0, 127.2, 129.5, 129.9, 130.0, 137.3, 142.5, 160.2, 162.6, 163.5, 180.1, 188.8; IR (ATR KBr cell, cm−1) 721, 1197, 1440, 1620, 1728, 2890, 3188; LC-MS calcd m/z: 468 found 469 [(M + 1)]+. Anal. calcd for C25H25FN2O2S2: C, 64.08; H, 5.38; N, 5.98; found: C, 64.03; H, 5.36; N, 5.95.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-bromophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4k. Off white solid; isolated yield 0.303 g (94%); M. Pt. 154–156 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.78 (m, 4H), 2.14 (s, 3H), 2.22 (s, 3H), 2.33–2.48 (m, 2H), 3.65–3.70 (m, 1H), 4.11 (d, J = 12 Hz, 1H), 4.28 (t, J = 11.6 Hz, 1H), 5.56 (s, 1H), 6.78 (d, J = 7.2 Hz, 1H), 6.94 (t, J = 7.2 Hz, 1H), 7.12–7.19 (m, 3H), 7.37 (d, J = 7.6 Hz, 1H), 7.51 (d, J = 8 Hz, 1H), 7.61 (d, J = 8 Hz, 1H), 10.49 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.3, 30.1, 47.5, 49.4, 67.9, 73.3, 73.5, 110.3, 111.6, 121.7, 125.5, 126.0, 128.6, 128.7, 128.8, 129.7, 133.2, 140.0, 142.7, 163.8, 179.8, 188.5; IR (ATR KBr cell, cm−1) 713, 1197, 1425, 1602, 1716, 2918, 3200; LC-MS calcd m/z: 529 found 530 [(M + 1)]+. Anal. calcd for C25H25BrN2O2S2: C, 56.71; H, 4.76; N, 5.29; found: C, 56.68; H, 4.70; N, 5.24.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-bromophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4l. Off white solid; isolated yield 0.319 g (99%); M. Pt. 158–160 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.70 (m, 4H), 1.78–1.82 (m, 2H), 2.15 (s, 3H), 2.25 (s, 3H), 2.31–2.36 (m, 1H), 2.39–2.45 (m, 1H), 3.70 (m, 2H), 3.95–3.98 (m, 1H), 5.60 (s, 1H), 6.77 (d, J = 7.6 Hz, 1H), 6.91 (td, J = 0.8 Hz, 7.6 Hz, 1H), 7.17 (td, J = 1.2 Hz, 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.51 (d, J = 8.4 Hz, 2H), 10.49 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.1, 16.3, 27.0, 29.9, 47.2, 50.4, 67.4, 72.2, 73.0, 109.6, 111.6, 119.6, 121.1, 125.5, 126.8, 129.1, 130.1, 131.4, 140.3, 142.1, 163.2, 179.6, 188.3; IR (ATR KBr cell, cm−1) 749, 1128, 1428, 1614, 1720, 2829, 3153; LC-MS calcd m/z: 529 found 530 [(M + 1)]+. Anal. calcd for C25H25BrN2O2S2: C, 56.71; H, 4.76; N, 5.29; found: C, 56.65; H, 4.73; N, 5.26.
4-(2′-(3,3-Bis(methylthio)acryloyl)-2-oxo-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizine]-1′-yl)benzonitrile 4m. Off white solid; isolated yield 0.304 g (88%); M. Pt. 168 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.68–1.80 (m, 4H), 2.15 (s, 3H), 2.24 (s, 3H), 2.32–2.36 (m, 1H), 3.75–3.84 (m, 2H), 4.02 (d, J = 11.6 Hz, 1H), 5.58 (s, 1H), 6.77 (d, J = 7.2 Hz, 1H), 6.91 (t, J = 7.6 Hz, 1H), 7.17 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 7.6 Hz, 1H), 7.61 (d, J = 8 Hz, 2H), 7.78 (d, J = 8 Hz, 1H), 10.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.2, 16.3, 27.0, 29.8, 47.3, 51.0, 67.3, 72.1, 73.0, 109.4, 109.7, 111.4, 119.0, 121.2, 125.4, 126.9, 129.0, 129.3, 132.5, 142.1, 146.9, 163.5, 179.6, 188.1; IR (ATR KBr cell, cm−1) 709, 1018, 1322, 1490, 1608, 1704, 2230, 2860, 3110; LC-MS calcd m/z: 475 found 476 [(M + 1)]+. Anal. calcd for C26H25N3O2S2: C, 65.66; H, 5.30; N, 8.83; found: C, 65.61; H, 5.27; N, 8.78.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2,4-dichlorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4n. Off white solid; isolated yield 0.293 g (90%); M. Pt. 126–128 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.71 (m, 2H), 1.77–1.84 (m, 2H), 2.16 (s, 3H), 2.25 (s, 3H), 2.34–2.45 (m, 2H), 3.65–3.71 (m, 1H), 4.13 (d, J = 11.8 Hz, 1H), 4.20–4.25 (m, 1H), 5.57 (s, 1H), 6.79 (d, J = 7.6 Hz, 1H), 6.92–6.96 (m, 1H), 7.12–7.23 (m, 2H), 7.41 (dd, J = 2.16 Hz, 8.44 Hz, 1H), 7.57–7.65 (m, 1H), 7.69–7.71 (m, 1H), 10.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.3, 30.3, 46.6, 47.4, 67.6, 72.8, 73.2, 110.2, 111.6, 114.2, 121.8, 125.8, 126.7, 128.3, 129.3, 129.9, 130.1, 131.8, 132.1, 133.9, 135.0, 137.6, 142.6, 164.0, 166.3, 179.8, 182.6, 188.3; IR (ATR KBr cell, cm−1) 758, 860, 1498, 1616, 1723, 2878, 2930, 3101; LC-MS calcd m/z: 519 found 520 [(M + 1)]+. Anal. calcd for C25H24ClN2O2S2: C, 57.80; H, 4.66; N, 5.39; found: C, 57.78; H, 4.63; N, 5.35.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2,4-difluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4o. Off white solid; isolated yield 0.309 g (91%); M. Pt. 140–142 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.59–1.70 (m, 2H), 1.79–1.85 (m, 2H), 2.16 (s, 3H), 2.27 (s, 3H), 2.32–2.45 (m, 2H), 3.74–3.78 (m, 1H), 3.77–3.92 (m, 1H), 4.14 (d, J = 12 Hz, 1H), 5.60 (s, 1H), 6.78 (dd, J = 4.4 Hz, 7.6 Hz, 1H), 6.93 (t, J = 7.2 Hz, 1H), 7.07 (td, J = 2 Hz, 8.4 Hz, 1H), 7.15–7.22 (m, 3H), 7.57 (q, J = 8.4 Hz, 1H), 10.53 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 19.0, 27.4, 30.6, 44.6, 47.5, 56.5, 66.5, 71.4, 73.3, 104.1, 104.4, 104.6, 110.1, 112.0, 121.7, 123.9, 124.0, 125.8, 127.0, 129.6, 130.8, 130.9, 142.5, 159.8, 160.2, 163.8, 179.9, 188.5; IR (ATR KBr cell, cm−1) 748, 846, 1132, 1425, 1502, 1616, 1708, 2870, 3080, 3190; LC-MS calcd m/z: 486 found 487 [(M + 1)]+. Anal. calcd for C25H24F2N2O2S2: C, 61.71; H, 4.97; N, 5.76; found: C, 61.71; H, 4.95; N, 5.70.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3,4-dimethoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4p. Off white solid; isolated yield 0.296 g (90%); M. Pt. 148 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.69 (m, 2H), 1.76–1.81 (m, 2H), 2.14 (s, 3H), 2.22 (s, 3H), 2.31–2.40 (m, 2H), 3.59–3.67 (m, 1H), 3.70 (s, 3H), 3.78 (s, 3H), 3.91 (d, J = 12 Hz, 1H), 5.64 (s, 1H), 6.75 (d, J = 7.6 Hz, 1H), 6.87–6.92 (m, 3H), 7.15 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 7.6 Hz, 1H), 10.39 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 27.4, 30.4, 47.6, 51.2, 56.0, 56.4, 67.8, 72.8, 73.5, 110.0, 112.1, 112.3, 112.5, 119.9, 121.4, 126.2, 127.2, 129.4, 133.5, 142.6, 148.0, 149.2, 163.2, 180.1, 189.2; IR (ATR KBr cell, cm−1) 720, 1081, 1420, 1606, 1750, 2158, 2300, 2950, 3112; LC-MS calcd m/z: 510 found 511 [(M + 1)]+. Anal. calcd for C27H30N2O4S2: C, 63.50; H, 5.92; N, 5.49; found: C, 63.46; H, 5.89; N, 5.46.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromo-2-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4q. Off white solid; isolated yield 0.268 g (86%); M. Pt. 178 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.76 (m, 4H), 2.14 (s, 4H), 2.25 (s, 4H), 3.80–3.84 (m, 2H), 3.84 (s, 3H), 3.96–4.02 (m, 1H), 4.22 (d, J = 12 Hz, 1H), 5.64 (s, 1H), 6.76 (d, J = 7.2 Hz, 1H), 6.91 (t, J = 7.6 Hz, 1H), 6.97 (d, J = 8.8 Hz, 1H), 7.14–7.18 (m, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.50 (s, 1H), 10.44 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 13.5, 15.1, 25.8, 29.0, 44.4, 55.5, 64.6, 70.2, 71.8, 109.0, 111.1, 111.6, 113.2, 120.4, 125.8, 128.4, 129.5, 130.0, 130.6, 141.6, 156.4, 162.2, 178.9, 188.2; IR (ATR KBr cell, cm−1) 760, 890, 1101, 1435, 1607, 1744, 2188, 2998, 3014; LC-MS calcd m/z: 559 found 560 [(M + 1)]+. Anal. calcd for C26H28N2O3S2: C, 55.81; H, 4.86; N, 5.01; found: C, 55.76; H, 4.83; N, 4.99.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-chloro-5-nitrophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4r. Off white solid; isolated yield 0.283 g (88%); M. Pt. 152–154 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.71 (m, 2H), 1.81–1.85 (m, 1H), 2.17 (s, 3H), 2.23 (s, 3H), 2.38–2.43 (m, 1H), 2.49–2.53 (m, 1H), 3.81–3.86 (m, 1H), 4.19 (d, J = 11.6 Hz, 1H), 4.33–4.40 (m, 1H), 5.58 (s, 1H), 6.81 (d, J = 7.6 Hz, 1H), 6.95 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.2 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 8.08 (dd, J = 2.4 Hz, 8.8 Hz, 1H), 8.40 (d, J = 2.4 Hz, 1H), 10.63 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.1, 30.0, 47.3, 47.6, 67.8, 72.7, 73.3, 110.2, 111.9, 121.8, 123.3, 123.8, 125.7, 126.9, 129.8, 131.4, 140.7, 141.0, 142.6, 147.3, 163.9, 179.8, 188.4; IR (ATR KBr cell, cm−1) 740, 1120, 1498, 1520, 1640, 1733, 2198, 2940, 3184; LC-MS calcd m/z: 530 found 431 [(M + 1)]+. Anal. calcd for C25H24ClN3O4S2: C, 56.65; H, 4.56; N, 7.93; found: C, 56.61; H, 4.53; N, 7.88.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3-bromo-4-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4s. Off white solid; isolated yield 0.284 g (90%); M. Pt. 116–118 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.68 (m, 2H), 1.76–1.81 (m, 2H), 2.15 (s, 3H), 2.24 (s, 3H), 2.33–2.35 (m, 1H), 2.38–2.45 (m, 1H), 3.72–3.76 (m, 2H), 3.91–3.94 (m, 1H), 5.59 (s, 1H), 6.76 (d, J = 7.6 Hz, 1H), 6.89 (t, J = 7.6 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 7.6 Hz, 1H), 7.32 (t, J = 8.8 Hz, 1H), 7.42–7.45 (m, 1H), 7.73–7.75 (m, 1H), 10.46 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.3, 30.0, 47.7, 50.3, 67.8, 72.5, 73.4, 108.2, 110.0, 112.1, 117.1, 121.5, 125.9, 127.2, 129.2, 129.3, 129.6, 133.3, 139.6, 142.5, 156.3, 158.7, 163.6, 180.0, 188.7; IR (ATR KBr cell, cm−1) 750, 1047, 1136, 1246, 1475, 1490, 1618, 1716, 2882, 3253; LC-MS calcd m/z: 547 found 548 [(M + 1)]+. Anal. calcd for C25H24BrFN2O2S2: C, 54.84; H, 4.42; N, 5.12; found: C, 54.80; H, 4.39; N, 5.09.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromothiophen-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4t. Off white solid; isolated yield 0.297 g (93%); M. Pt. 128–130 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.75 (m, 2H), 1.78–1.82 (m, 1H), 1.88–1.93 (m, 1H), 2.19 (s, 3H), 2.28 (s, 3H), 2.32–2.44 (m, 2H), 3.76–3.82 (m, 2H), 3.91–3.94 (m, 1H), 5.61 (s, 1H), 6.76 (d, J = 7.6 Hz, 1H), 6.85–6.91 (m, 2H), 7.05 (d, J = 3.72 Hz, 1H), 7.13–7.19 (m, 2H), 10.48 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.3, 30.3, 46.8, 47.6, 68.7, 72.1, 73.5, 109.3, 110.1, 111.7, 121.5, 125.5, 126.0, 127.3, 129.6, 130.5, 142.4, 146.4, 164.8, 176.8; IR (ATR KBr cell, cm−1) 723, 983, 1421, 1614, 1720, 2900, 3148; LC-MS calcd m/z: 535 found 536 [(M + 1)]+. Anal. calcd for C23H23BrN2O2S3: C, 51.58; H, 4.33; N, 5.23; found: C, 51.53; H, 4.30; N, 5.16.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromopyridin-3-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4u. Off white solid; isolated yield 0.296 g (92%); M. Pt. 156–158 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.72 (m, 2H), 1.78–1.84 (m, 2H), 2.16 (s, 3H), 2.25 (s, 3H), 2.33–2.38 (m, 1H), 2.41–2.49 (m, 1H), 3.75–3.82 (m, 2H), 4.01–4.06 (m, 1H), 5.61 (s, 1H), 6.78 (d, J = 7.56 Hz, 1H), 6.92 (dd, J = 0.72 Hz, 7.52 Hz, 1H), 7.18 (dd, J = 1 Hz, 7.68 Hz, 1H), 7.26 (t, J = 7.44 Hz, 1H), 8.11 (t, J = 2.4 Hz, 1H), 8.56 (d, J = 2.2 Hz, 1H), 8.62 (d, J = 1.8 Hz, 1H), 10.50 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.2, 29.8, 47.7, 48.5, 67.4, 72.1, 73.4, 110.1, 112.0, 120.8, 121.6, 125.8, 129.7, 138.1, 139.2, 142.5, 148.6, 149.0, 163.7, 179.8, 188.6; IR (ATR KBr cell, cm−1) 712, 1066, 1188, 1386, 1421, 1612, 1713, 2945, 3259; LC-MS calcd m/z: 530 found 531 [(M + 1)]+. Anal. calcd for C24H24BrN3O2S2: C, 54.34; H, 4.56; N, 7.92; found: C, 54.29; H, 4.51; N, 7.90.
2′-(3,3-Bis(methylthio)acryloyl)-5-methoxy-1′-o-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ba. Off white solid; isolated yield 0.360 g (96%); M. Pt. 200–202 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.71–1.82 (m, 2H), 1.83–1.87 (m, 2H), 2.18 (s, 3H), 2.22 (s, 3H), 2.37–2.42 (m, 1H), 2.55 (s, 3H), 3.71 (s, 3H), 3.76–3.81 (m, 1H), 3.94 (t, J = 9.6 Hz, 1H), 4.04 (d, J = 11.2 Hz, 1H), 5.54 (s, 1H), 6.70–6.72 (m, 2H), 6.78 (dd, J = 2.4 Hz, 8.4 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H), 7.15–7.21 (m, 2H), 7.40 (d, J = 8 Hz, 1H), 10.38 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.5, 20.2, 27.5, 30.6, 46.3, 47.4, 55.8, 69.7, 73.5, 73.8, 110.3, 112.2, 113.8, 114.3, 126.4, 126.6, 126.8, 127.5, 130.6, 135.9, 137.0, 139.6, 154.6, 163.3, 180.0, 189.1; IR (ATR KBr cell, cm−1) 731, 1197, 1489, 1728, 2684, 3416; LC-MS calcd m/z: 494 found 495 [(M + 1)]+. Anal. calcd for C27H30N2O3S2: C, 65.56; H, 6.11; N, 5.66; found: C, 65.51; H, 6.08; N, 5.64.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-isopropylphenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4da. Off white solid; isolated yield 0.325 g (91%); M. Pt. 134–136 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.18 (d, J = 6.8 Hz, 6H), 1.66–1.67 (m, 2H), 1.76–1.82 (m, 2H), 2.36 (s, 1H), 2.43–2.45 (m, 1H), 2.80–2.87 (m, 1H), 3.59–3.76 (m, 5H), 3.95 (d, J = 12 Hz, 1H), 5.61 (s, 1H), 6.68 (d, J = 8.4 Hz, 1H), 6.74–6.78 (m, 2H), 7.18 (d, J = 8 Hz, 2H), 7.31 (d, J = 7.6 Hz, 2H), 10.26 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 13.5, 23.3, 26.3, 29.3, 32.4, 46.5, 50.0, 55.0, 66.9, 71.7, 72.6, 109.1, 112.9, 113.6, 125.8, 127.0, 135.0, 135.2, 137.4, 145.9, 153.6, 178.9, 188.1; IR (ATR KBr cell, cm−1) 750, 1136, 1480, 1720, 2980, 3500; LC-MS calcd m/z: 522 found 523 [(M + 1)]+. Anal. calcd for C29H34N2O3S2: C, 66.63; H, 6.56; N, 5.36; found: C, 66.58; H, 6.53; N, 5.30.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-ethoxyphenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ea. Off white solid; isolated yield 0.32 g (92%); M. Pt. 150 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.32 (t, J = 6.8 Hz, 1H), 1.66–1.70 (m, 2H), 1.76–1.86 (m, 2H), 2.18 (s, 3H), 2.20 (s, 3H), 2.33–2.37 (m, 1H), 2.43–2.47 (m, 1H), 3.60–3.65 (m, 1H), 3.71 (s, 4H), 3.91 (d, J = 11.6 Hz, 1H), 4.00 (q, J = 6.8 Hz, 1H), 5.63 (s, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.75–6.77 (m, 1H), 6.80–6.81 (m, 1H), 6.87 (d, J = 8.8 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 10.29 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 15.2, 16.7, 27.4, 30.3, 47.6, 50.7, 56.0, 63.4, 68.0, 72.8, 73.7, 79.6, 110.1, 112.4, 113.8, 114.7, 114.9, 127.5, 129.1, 132.8, 136.0, 154.6, 157.7, 163.1, 180.0, 189.2; IR (ATR KBr cell, cm−1) 780, 1190, 1429, 1700, 2888, 3200, 3540; LC-MS calcd m/z: 524 found 525 [(M + 1)]+. Anal. calcd for C28H32N2O4S2: C, 64.09; H, 6.15; N, 5.34; found: C, 64.07; H, 6.11; N, 5.29.
2′-(3,3-Bis(methylthio)acryloyl)-5-methoxy-1′-(3-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ga. Off white solid; isolated yield 0.346 g (95%); M. Pt. 196 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.06–1.85 (m, 4H), 2.18 (s, 3H), 2.24 (s, 3H), 3.67–3.70 (m, 4H), 3.73–3.76 (m, 4H), 3.95–3.96 (m, 1H), 5.66 (s, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.75–6.83 (m, 3H), 6.97–6.99 (m, 2H), 7.25 (t, J = 8 Hz, 1H), 10.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 27.3, 30.3, 47.6, 51.4, 55.4, 56.0, 56.5, 67.8, 72.7, 73.7, 110.2, 112.2, 112.4, 113.8, 114.3, 114.8, 120.2, 127.5, 130.0, 136.0, 142.9, 154.7, 159.8, 163.3, 180.0, 189.2; IR (ATR KBr cell, cm−1) 738, 1303, 1489, 1712, 3431; LC-MS calcd m/z: 510 found 511 [(M + 1)]+. Anal. calcd for C27H30N2O4S2: C, 63.50; H, 5.92; N, 5.49; found: C, 63.44; H, 5.88; N, 5.43.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-fluorophenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ia. Off white solid; isolated yield 0.346 g (93%); M. Pt. 206–208 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.61–1.81 (m, 4H), 2.15 (s, 3H), 2.23 (s, 3H), 2.35–2.48 (m, 1H), 3.77 (m, 3H), 3.89 (t, J = 10 Hz, 1H), 4.13 (d, J = 12 Hz, 1H), 5.59 (s, 1H), 6.67–6.76 (m, 3H), 7.12–7.25 (m, 3H), 7.51 (t, J = 6.8 Hz, 1H), 10.31 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 13.6, 15.4, 26.5, 29.6, 43.9, 46.5, 55.0, 65.6, 70.6, 72.8, 109.4, 110.9, 112.8, 113.5, 114.9, 115.2, 124.2, 126.4, 126.7, 127.9, 128.8, 135.0, 153.7, 155.7, 158.1, 162.7, 178.9, 187.7; IR (ATR KBr cell, cm−1) 732, 1450, 1730, 2918, 3024, 3450; LC-MS calcd m/z: 498 found 499 [(M + 1)]+. Anal. calcd for C26H27FN2O3S2: C, 62.63; H, 5.46; N, 5.62; found: C, 62.60; H, 5.41; N, 5.58.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-bromophenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ka. Off white solid; isolated yield 0.313 g (92%); M. Pt. 212–214 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.68 (m, 2H), 1.78–1.84 (m, 2H), 2.18 (s, 3H), 2.24 (s, 3H), 2.34–2.37 (m, 1H), 2.43–2.48 (m, 1H), 3.60–3.65 (m, 1H), 3.69–3.73 (m, 4H), 3.91 (d, J = 12 Hz, 1H), 5.64 (s, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.75–6.81 (m, 2H), 6.89 (d, J = 2.8 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H), 10.29 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.3, 30.0, 47.4, 49.3, 55.8, 67.9, 73.4, 73.7, 110.5, 111.9, 113.9, 114.0, 125.5, 127.3, 128.7, 128.8, 133.2, 136.0, 140.0, 154.6, 163.7, 179.6, 188.6; IR (ATR KBr cell, cm−1) 742, 1199, 1489, 1693, 1726, 2860, 3263; LC-MS calcd m/z: 559 found 560 [(M + 1)]+. Anal. calcd for C26H27BrN2O3S2: C, 55.81; H, 4.86; N, 5.01; found: C, 55.78; H, 4.81; N, 4.98.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-bromophenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4la. Off white solid; isolated yield 0.319 g (94%); M. Pt. 186–188 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.69 (m, 2H), 1.77–1.84 (m, 2H), 2.17 (s, 3H), 2.23 (s, 3H), 2.32–2.51 (m, 2H), 3.67–3.71 (m, 5H), 3.92 (d, J = 11.5 Hz, 1H), 5.60 (s, 1H), 6.68 (d, J = 8.36 Hz, 1H), 6.74–6.76 (m, 1H), 6.80 (d, J = 2.44 Hz, 1H), 7.37 (dd, J = 1.76 Hz, 6.88 Hz, 2H), 7.49–7.51 (m, 2H), 10.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.6, 27.3, 30.1, 47.6, 50.7, 56.0, 56.5, 67.8, 72.5, 73.6, 110.2, 112.3, 114.0, 120.0, 127.3, 130.5, 131.8, 136.0, 140.7, 154.7, 163.4, 179.9, 188.9; IR (ATR KBr cell, cm−1) 745, 1145, 1428, 1690, 1718, 2875, 2928, 3198, 3435; LC-MS calcd m/z: 559 found 560 [(M + 1)]+. Anal. calcd for C26H27BrN2O3S2: C, 55.81; H, 4.86; N, 5.01; found: C, 55.75; H, 4.80; N, 4.95.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3-bromo-4-fluorophenyl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4sa. Off white solid; isolated yield 0.299 g (90%); M. Pt. 120–122 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.68–1.84 (m, 4H), 2.19 (s, 3H), 2.26 (s, 3H), 2.37–2.51 (m, 2H), 3.71–3.75 (m, 5H), 3.92–3.95 (m, 1H), 5.63 (s, 1H), 6.70 (d, J = 8.08 Hz, 1H), 6.77 (d, J = 8.28 Hz, 1H), 6.84 (s, 1H), 7.34 (d, J = 8.48 Hz, 1H), 7.46 (s, 1H), 7.80 (d, J = 6.36 Hz, 1H), 10.34 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.2, 29.9, 47.7, 50.1, 67.8, 72.6, 73.7, 79.7, 108.2, 110.2, 112.3, 114.0, 114.7, 117.3, 127.3, 129.2, 133.4, 139.6, 139.6, 154.3, 156.3, 158.7, 163.5, 179.8, 188.9; IR (ATR KBr cell, cm−1) 755, 1480, 1720, 2918, 3270, 3578; LC-MS calcd m/z: 577 found 578 [(M + 1)]+. Anal. calcd for C26H26BrN2O3S2: C, 54.07; H, 4.54; N, 4.85; found: C, 54.03; H, 4.51; N, 4.79.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromothiophen-2-yl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ta. Off white solid; isolated yield 0.334 g (99%); M. Pt. 154 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.68–1.77 (m, 2H), 1.81–1.93 (m, 2H), 2.18 (s, 3H), 2.25 (s, 3H), 2.32–2.47 (m, 2H), 3.44 (s, 3H), 3.74–3.87 (m, 2H), 3.93–3.98 (m, 1H), 5.64 (s, 1H), 6.67–6.70 (m, 1H), 6.75–6.77 (m, 2H), 6.87–6.93 (m, 1H), 7.07 (d, J = 3.6 Hz, 1H), 10.34 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.3, 30.1, 46.7, 47.6, 56.1, 68.6, 72.1, 73.7, 109.3, 110.3, 112.0, 114.1, 114.7, 126.1, 126.9, 130.5, 135.9, 146.5, 154.7, 164.1, 179.6, 188.4; IR (ATR KBr cell, cm−1) 748, 1078, 1343, 1470, 1628, 1709, 2918, 3440; LC-MS calcd m/z: 565 found 566 [(M + 1)]+. Anal. calcd for C24H25BrN2O3S3: C, 50.97; H, 4.46; N, 4.95; found: C, 50.91; H, 4.43; N, 4.90.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromopyridin-3-yl)-5-methoxy-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ua. Off white solid; isolated yield 0.312 g (92%); M. Pt. 208–210 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.67–1.81 (m, 2H), 1.83–1.87 (m, 2H), 2.18 (s, 3H), 2.24 (s, 3H), 2.35–2.39 (m, 1H), 2.45–2.51 (m, 1H), 3.69 (s, 3H), 3.73–3.79 (m, 2H), 4.02 (d, J = 11.3 Hz, 1H), 5.62 (s, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.75–6.78 (m, 1H), 6.83 (d, J = 2.4 Hz, 1H), 8.13 (t, J = 2 Hz, 1H), 8.55 (d, J = 2.2 Hz, 1H), 8.63 (d, J = 1.76 Hz, 1H), 10.36 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.6, 27.2, 29.7, 47.7, 48.4, 56.1, 67.4, 72.1, 73.6, 110.2, 112.3, 114.1, 120.7, 127.1, 135.9, 138.2, 139.2, 148.6, 148.9, 154.8, 163.9, 179.7, 188.7; IR (ATR KBr cell, cm−1) 704, 823, 1016, 1203, 1489, 1645, 1716, 2868, 3024, 3417, 3514; LC-MS calcd m/z: 560 found 561 [(M + 1)]+. Anal. calcd for C25H26BrN3O3S2: C, 53.57; H, 5.68; N, 7.50; found: C, 53.52; H, 5.64; N, 7.48.
2′-(3,3-Bis(methylthio)acryloyl)-5-nitro-1′-o-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4bb. Off white solid; isolated yield 0.347 g (90%); M. Pt. 200–204 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.79 (m, 2H), 1.82–1.91 (m, 2H), 2.14 (s, 3H), 2.21 (s, 3H), 2.49–2.59 (m, 4H), 3.85–3.90 (m, 1H), 4.00 (t, J = 9.2 Hz, 1H), 4.09 (d, J = 11.2 Hz, 1H), 5.56 (s, 1H), 7.00 (d, J = 8.4 Hz, 1H), 7.01–7.11 (m, 1H), 7.16–7.23 (m, 2H), 7.45 (d, J = 7.2 Hz, 1H), 7.92 (d, J = 7.2 Hz, 1H), 8.19 (dd, J = 2 Hz, 8.4 Hz, 1H), 11.28 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 20.1, 27.4, 30.3, 46.5, 47.6, 69.9, 73.0, 73.5, 110.3, 112.0, 122.3, 126.7, 126.8, 126.9, 127.0, 127.2, 130.7, 137.0, 138.8, 142.1, 149.2, 164.9, 180.5, 189.0; IR (ATR KBr cell, cm−1) 749, 1140, 1480, 1600, 1728, 2970, 3300; LC-MS calcd m/z: 508 found 509 [(M + 1)]+. Anal. calcd for C26H27N3O4S2: C, 61.27; H, 5.34; N, 8.25; found; C, 61.23; H, 5.29; N, 8.20.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-isopropylphenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4db. Off white solid; isolated yield 0.335 g (91%); M. Pt. 196–198 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.18 (d, J = 6.88 Hz, 6H), 1.70–1.73 (m, 2H), 1.81–1.87 (m, 2H), 2.14 (s, 3H), 2.21 (s, 3H), 2.39–2.43 (m, 1H), 2.47–2.52 (m, 2H), 2.80–2.87 (m, 1H), 3.67–3.73 (m, 1H), 3.78–3.83 (m, 1H), 4.00 (d, J = 11.8 Hz, 1H), 5.66 (s, 1H), 6.98 (d, J = 8.64 Hz, 1H), 7.20 (d, J = 8.16 Hz, 2H), 7.35 (d, J = 8.16 Hz, 2H), 7.99 (d, J = 2.28 Hz, 1H), 8.17 (dd, J = 2.32 Hz, 8.64 Hz, 1H), 11.28 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 24.3, 24.4, 27.4, 30.1, 33.5, 47.8, 51.2, 68.3, 72.9, 73.1, 110.3, 112.0, 122.3, 126.9, 127.0, 127.2, 128.2, 137.8, 142.1, 149.2, 164.9, 180.6, 189.0; IR (ATR KBr cell, cm−1) 748, 1368, 1445, 1630, 1700, 2970, 3320; LC-MS calcd m/z: 537 found 538 [(M + 1)]+. Anal. calcd for C28H31N3O4S2: C, 62.54; H, 5.81; N, 7.81; found: C, 62.48; H, 5.78; N, 7.80.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-ethoxyphenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4eb. Off white solid; isolated yield 0.341 g (93%); M. Pt. 190–192 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.31 (t, J = 6.8 Hz, 3H), 1.68–1.76 (m, 2H), 1.78–1.88 (m, 2H), 2.14 (s, 3H), 2.24 (s, 3H), 2.38–2.43 (m, 1H), 2.49–2.54 (m, 1H), 3.65–3.71 (m, 1H), 3.76–3.81 (m, 1H), 3.94–4.01 (m, 3H), 5.68 (s, 1H), 6.88 (d, J = 8.8 Hz, 2H), 6.98 (d, J = 8.4 Hz, 1H), 7.33 (d, J = 8.8 Hz, 2H), 7.99 (d, J = 2 Hz, 1H), 8.17 (dd, J = 2 Hz, 8.4 Hz, 1H), 11.20 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 15.2, 16.7, 27.4, 30.0, 47.8, 50.7, 63.4, 68.4, 72.9, 73.0, 110.3, 112.1, 115.0, 122.4, 126.9, 127.2, 129.2, 132.1, 142.1, 149.2, 157.8, 164.8, 180.6, 189.1; IR (ATR KBr cell, cm−1) 746, 910, 1049, 1332, 1483, 1739, 2972, 3354; LC-MS calcd m/z: 538 found 539 [(M + 1)]+. Anal. calcd for C27H29N3O5S2: C, 60.09; H, 5.42; N, 7.79; found: C, 60.05; H, 5.39; N, 7.72.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3-methoxyphenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4gb. Off white solid; isolated yield 0.33 g (88%); M. Pt. 162 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.69–1.77 (m, 2H), 1.79–1.86 (m, 2H), 2.14 (s, 3H), 2.24 (s, 3H), 2.40–2.50 (m, 1H), 2.55 (s, 1H), 3.71–3.85 (m, 5H), 4.01 (t, J = 12 Hz, 1H), 5.70 (s, 1H), 6.80 (d, J = 8 Hz, 2H), 6.97–7.01 (m, 3H), 7.26 (t, J = 7.6 Hz, 1H), 8.01 (s, 1H), 8.17 (dd, J = 1.2 Hz, 8.8 Hz, 1H), 11.21 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.3, 30.0, 47.8, 51.4, 55.5, 68.1, 72.9, 73.1, 110.3, 112.1, 112.5, 114.2, 120.2, 122.4, 127.0, 130.1, 142.1, 142.2, 149.3, 159.9, 165.0, 180.6, 189.1; IR (ATR KBr cell, cm−1) 720, 840, 1130, 1480, 1740, 2800, 3260; LC-MS calcd m/z: 525 found 526 [(M + 1)]+. Anal. calcd for C26H27N3O5S2: C, 59.41; H, 5.18; N, 7.99; C, 59.38; H, 5.11; N, 7.94.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-fluorophenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ib. Off white solid; isolated yield 0.352 g (92%); M. Pt. 206–208 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.84–1.90 (m, 4H), 2.14 (s, 3H), 2.26 (s, 3H), 2.42–2.45 (m, 1H), 2.49–2.52 (m, 1H), 3.85–3.89 (m, 1H), 3.93–3.98 (m, 1H), 4.21 (d, J = 11.68 Hz, 1H), 5.66 (s, 1H), 6.99 (d, J = 8.64 Hz, 1H), 7.00–7.21 (m, 2H), 7.26–7.31 (m, 1H), 7.58, (q, J = 7.8 Hz, 1H), 7.94 (d, J = 2.24 Hz, 1H), 8.18 (dd, J = 2.28 Hz, 8.64 Hz, 1H), 11.30, (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.6, 27.4, 30.3, 45.2, 47.7, 56.5, 68.8, 71.6, 73.0, 110.4, 111.6, 116.2, 122.2, 125.2, 126.7, 126.9, 127.1, 129.0, 127.1, 129.0, 129.9, 1421, 149.2, 159.9, 162.4, 165.3, 180.4, 188.5; IR (ATR KBr cell, cm−1) 710, 1170, 1300, 1490, 1748, 2800, 3400; LC-MS calcd m/z: 513 found 514 [(M + 1)]+. Anal. calcd for C25H24FN3O4S2: C, 58.46; H, 4.71; N, 8.18; found: C, 58.42; H, 4.66; N, 8.15.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(2-bromophenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4kb. Off white solid; isolated yield 0.311 g (89%); M. Pt. 226–228 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.83–1.88 (m, 4H), 2.15 (s, 3H), 2.21 (s, 3H), 2.44–2.55 (m, 1H), 3.67–3.82 (m, 1H), 4.17 (d, J = 11.6 Hz, 1H), 4.29–4.35 (m, 1H), 5.62 (s, 1H), 7.01 (d, J = 8.68 Hz, 1H), 7.19 (td, J = 1.56 Hz, 8 Hz, 1H), 7.42 (td, J = 1.04 Hz, 7.56 Hz, 1H), 7.61–7.66 (m, 2H), 7.97 (d, J = 2.28 Hz, 1H), 8.20 (d, J = 2.24 Hz, 8.6 Hz, 1H), 11.35 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.2, 29.8, 47.7, 49.5, 55.4, 68.1, 73.0, 73.5, 110.5, 111.6, 122.0, 125.4, 127.0, 127.1, 128.9, 129.0, 129.1, 133.3, 139.3, 142.0, 149.3, 165.4, 180.1, 188.5; IR (ATR KBr cell, cm−1) 752, 1130, 1494, 1710, 2879, 3387; LC-MS calcd m/z: 574 found 575 [(M + 1)]+. Anal. calcd for C25H24BrN3O4S2: C, 52.26; H, 4.21; N, 7.31; found: C, 52.22; H, 4.16; N, 7.29.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(4-bromophenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4lb. Off white solid; isolated yield 0.325 g (93%); M. Pt. 196–198 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.73–1.78 (m, 2H), 1.80–1.78 (m, 2H), 2.14 (s, 3H), 2.25 (s, 3H), 2.39–2.44 (m, 1H), 2.48–2.55 (m, 1H), 3.73–3.81 (m, 2H), 3.99 (d, J = 11.28 Hz, 1H), 5.66 (s, 1H), 6.98 (d, J = 8.68 Hz, 1H), 7.42 (d, J = 8.48 Hz, 2H), 7.52 (d, J = 8.44 Hz, 2H), 8.00 (d, J = 2.28 Hz, 1H), 8.17 (dd, J = 2.24 Hz, 8.6 Hz, 1H), 11.27 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.3, 29.8, 47.8, 50.8, 68.1, 72.6, 73.0, 110.3, 111.9, 120.3, 122.5, 126.9, 127.0, 130.6, 131.9, 140.0, 142.2, 149.2, 165.1, 180.5, 188.8; IR (ATR KBr cell, cm−1) 760, 1400, 1498, 1760, 2800, 3117, 3289; LC-MS calcd m/z: 574 found 575 [(M + 1)]+. Anal. calcd for C25H24BrN3O4S2: C, 52.26; H, 4.21; N, 7.31; found: C, 52.20; H, 4.18; N, 7.29.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(3-bromo-4-fluorophenyl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4sb. Off white solid; isolated yield 0.307 g (90%); M. Pt. 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.85 (m, 4H), 2.14 (s, 3H), 2.24 (s, 3H), 2.42–2.48 (m, 1H), 2.51–2.53 (m, 1H), 3.80 (d, J = 7.2 Hz, 2H), 3.97 (s, 1H), 5.67 (s, 1H), 6.97 (d, J = 8.4 Hz, 1H), 7.33 (t, J = 8.8 Hz, 1H) 7.47–7.50 (m, 1H), 7.82–7.84 (m, 1H), 8.00 (s, 1H), 8.16 (dd, J = 1.6 Hz, 8.8 Hz, 1H), 11.20 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.1, 29.5, 47.9, 50.1, 68.1, 72.7, 73.0, 108.3, 108.5, 110.3, 112.1, 117.4, 122.5, 127.0, 129.4, 133.4, 138.9, 142.2, 149.2, 156.4, 158.8, 165.1, 180.5, 188.8; IR (ATR KBr cell, cm−1) 740, 1280, 1640, 1718, 2300, 3088, 3421; LC-MS calcd m/z: 592 found 593 [(M + 1)]+. Anal. calcd for C25H23BrFN3O4S2: C, 50.68; H, 3.91; N, 7.09; found: C, 50.62; H, 3.88; N, 7.07.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromothiophen-2-yl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4tb. Off white solid; isolated yield 0.319 g (92%); M. Pt. 196–198 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.83–1.96 (m, 4H), 2.17 (s, 3H), 2.29 (s, 3H), 2.37–2.42 (m, 1H), 2.49–2.54 (m, 1H), 3.81–3.88 (m, 2H), 4.02–4.07 (m, 1H), 5.70 (s, 1H), 6.92 (d, J = 3.6 Hz, 1H), 6.97 (d, J = 8.4 Hz, 1H), 7.07 (d, J = 4 Hz, 1H), 7.97 (d, J = 2.4 Hz, 1H), 8.17 (dd, J = 2 Hz, 8.4 Hz, 1H), 11.24 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.8, 27.2, 29.7, 46.8, 47.9, 68.8, 72.2, 73.0, 109.6, 110.4, 111.8, 122.6, 126.4, 126.5, 127.1, 130.7, 142.2, 145.7, 149.1, 165.7, 180.3, 188.3; IR (ATR KBr cell, cm−1) 694, 732, 1093, 1336, 1481, 1624, 1716, 2854, 3227, 3042; LC-MS calcd m/z: 580 found 581 [(M + 1)]+. Anal. calcd for C23H22BrN3O4S3: C, 47.58; H, 3.82; N, 7.24; found: C, 47.51; H, 3.80; N, 7.19.
2′-(3,3-Bis(methylthio)acryloyl)-1′-(5-bromopyridin-3-yl)-5-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ub. Off white solid; isolated yield 0.328 g (94%); M. Pt. 216–218 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.70–1.91 (m, 4H), 2.15 (s, 3H), 2.25 (s, 3H), 2.40–2.49 (m, 1H), 2.50–2.59 (m, 1H), 3.82–3.89 (m, 2H), 4.12 (d, J = 11.2 Hz, 1H), 6.99 (d, J = 8.64 Hz, 1H), 8.01 (d, J = 2.24 Hz, 1H), 8.16–8.19 (m, 2H), 8.57 (d, J = 2.2 Hz, 1H), 8.67 (d, J = 1.8 Hz, 1H), 11.30 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.1, 29.3, 48.0, 48.4, 67.5, 72.3, 73.0, 110.3, 112.2, 120.8, 122.5, 126.7, 127.1, 133.3, 138.5, 142.3, 148.5, 149.1, 165.0, 180.3, 188.6; IR (ATR KBr cell, cm−1) 724, 906, 1480, 1708, 2800, 3058, 3423; LC-MS calcd m/z: 575 found 576 [(M + 1)]+. Anal. calcd for C24H23BrN4O4S2: C, 50.09; H, 4.03; N, 9.74; found: C, 50.02; H, 4.00; N, 9.70.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-o-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4bc. Off white solid; isolated yield 0.366 g (89%); M. Pt. 160–162 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.64–1.77 (m, 2H), 1.79–1.85 (m, 2H), 2.19 (s, 3H), 2.25 (s, 3H), 2.39–2.51 (m, 2H), 2.54 (s, 3H), 3.73–3.78 (m, 1H), 3.93 (t, J = 9.52 Hz, 1H), 4.07 (d, J = 11.62 Hz, 1H), 5.55 (s, 1H), 6.94 (s, 1H), 7.07–7.10 (m, 1H), 7.14–7.21 (m, 4H), 7.38 (d, J = 7.72 Hz, 1H), 10.67 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.5, 20.4, 27.7, 30.8, 46.4, 47.4, 69.6, 73.3, 73.7, 111.8, 112.9, 122.2, 124.4, 125.5, 126.5, 126.8, 130.7, 137.1, 139.3, 144.3, 163.9, 180.0, 188.8; IR (ATR KBr cell, cm−1) 727, 1134, 1325, 1494, 1602, 1710, 2854, 3159, 3320; LC-MS calcd m/z: 541 found 542 [(M + 1)]+. Anal. calcd for C26H27BrN2O2S2: C, 57.45; H, 5.01; N, 5.15; found: C, 57.40; H, 4.97; N, 5.13.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(4-isopropylphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4dc. Off white solid; isolated yield 0.344 g (88%); M. Pt. 170–172 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.18 (d, J = 6.8 Hz, 6H), 1.62–1.71 (m, 2H), 1.78–1.80 (m, 2H), 2.19 (s, 3H), 2.23 (s, 3H), 2.36–2.37 (m, 2H), 2.81–2.88 (m, 1H), 3.62–3.73 (m, 2H), 3.99 (d, J = 12 Hz, 1H), 5.63 (s, 1H), 6.93 (s, 1H), 7.11 (d, J = 7.6 Hz, 1H), 7.19–7.24 (m, 3H), 7.31 (d, J = 8 Hz, 2H), 10.62 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 20.4, 27.5, 30.5, 33.5, 47.6, 51.1, 67.8, 73.1, 73.3, 112.0, 112.8, 122.1, 124.1, 125.7, 127.0, 128.1, 129.0, 138.0, 144.3, 147.1, 164.0, 180.0, 188.9; IR (ATR KBr cell, cm−1) 734, 812, 1165, 1329, 1446, 1620, 1732, 3219, 3384; LC-MS calcd m/z: 569 found 570 [(M + 1)]+. Anal. calcd for C28H31BrN2O2S2: C, 58.84; H, 5.47; N, 4.90; found: C, 58.80; H, 5.41; N, 4.88.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(4-ethoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ec. Off white solid; isolated yield 0.355 g (91%); M. Pt. 200–202 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.32 (t, J = 6.88 Hz, 3H), 1.63–1.70 (m, 2H), 1.72–1.80 (m, 2H), 2.18 (s, 3H), 2.26 (s, 3H), 2.36–2.39 (m, 2H), 3.60–3.72 (m, 2H), 3.93 (d, J = 11.8 Hz, 1H), 4.00 (q, J = 6.92 Hz, 2H), 5.65 (s, 1H), 6.88 (d, J = 8.56 Hz, 2H), 6.92 (s, 1H), 7.11 (dd, J = 1.48 Hz, 7.92 Hz, 1H), 7.23 (d, J = 8.04 Hz, 1H), 7.29 (d, J = 8.56 Hz, 2H), 10.59 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 15.2, 16.7, 27.5, 30.4, 47.6, 50.6, 63.4, 67.9, 73.0, 73.2, 112.1, 112.8, 114.9, 122.1, 124.1, 125.6, 129.0, 132.5, 144.3, 157.8, 163.9, 180.0, 189.0; IR (ATR KBr cell, cm−1) 779, 1103, 1498, 1711, 2253, 3299; LC-MS calcd m/z: 573 found 574 [(M + 1)]+. Anal. calcd for C27H29BrN2O3S2: C, 56.54; H, 5.10; N, 4.88; found: C, 56.50; H, 5.08; N, 4.83.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(3-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4gc. Off white solid; isolated yield 0.367 g (92%); M. Pt. 150 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.70 (m, 2H), 1.76–1.82 (m, 2H), 2.19 (s, 3H), 2.24 (s, 3H), 2.35–2.36 (m, 2H), 3.64–3.75 (m, 5H), 3.98 (d, J = 11.6 Hz, 1H), 5.65 (s, 1H), 6.78–6.80 (m, 1H), 6.91–6.97 (m, 3H), 7.09–7.11 (m, 1H), 7.22–7.25 (m, 2H), 10.60 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 27.5, 30.4, 47.6, 51.4, 55.4, 67.6, 72.9, 73.3, 110.0, 112.0, 112.3, 112.8, 114.2, 120.1, 122.2, 124.1, 125.6, 129.0, 130.1, 142.6, 144.3, 159.8, 164.0, 179.9, 188.9; IR (ATR KBr cell, cm−1) 734, 1360, 1684, 1704, 3060, 3168, 3320; LC-MS calcd m/z: 559 found 560 [(M + 1)]+. Anal. calcd for C26H27BrN2O3S2: C, 55.81; H, 4.86; N, 5.01; found: C, 55.75; H, 4.83; N, 4.94.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(2-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4ic. Off white solid; isolated yield 0.363 g (89%); M. Pt. 134 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.60–1.71 (m, 2H), 1.78–1.83 (m, 2H), 2.18 (s, 3H), 2.27 (s, 3H), 2.32–2.38 (m, 2H), 3.72–3.77 (m, 1H), 3.85–3.90 (m, 1H), 4.17 (d, J = 12 Hz, 1H), 5.62 (s, 1H), 6.91 (s, 1H), 7.11–7.19 (m, 4H), 7.24–7.29 (m, 1H), 7.50 (d, J = 7.48 Hz, 1H), 10.75 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.6, 30.7, 45.1, 47.4, 66.4, 71.7, 73.2, 111.5, 112.9, 116.1, 122.2, 124.3, 125.2, 125.3, 127.2, 127.3, 128.8, 128.9, 129.8, 144.3, 159.9, 162.4, 164.3, 179.8, 188.4; IR (ATR KBr cell, cm−1) 760, 1128, 1444, 1620, 1700, 1780, 2980, 3420; LC-MS calcd m/z: 547 found 548 [(M + 1)]+. Anal. calcd for C25H24BrFN2O2S2: C, 54.84; H, 4.42; N, 5.12; C, 54.81; H, 4.38; N, 5.08.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(2-bromophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4kc. Off white solid; isolated yield 0.333 g (90%); M. Pt. 204–206 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.66–1.83 (m, 4H), 2.19 (s, 3H), 2.24 (s, 3H), 2.36–2.39 (m, 2H), 3.65–3.70 (m, 1H), 4.13 (d, J = 11.9 Hz, 2H), 4.19–4.25 (m, 1H), 5.58 (s, 1H), 6.93 (d, J = 1.8 Hz, 1H), 7.07 (d, J = 8.04 Hz, 1H), 7.14–7.18 (m, 2H), 7.39 (td, J = 1.08 Hz, 1.6 Hz, 1H), 7.53 (dd, J = 1.44 Hz, 7.92 Hz, 1H), 7.63 (dd, J = 1.2 Hz, 8 Hz, 1H), 10.65 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.6, 27.4, 30.1, 47.5, 49.4, 67.8, 73.1, 73.5, 111.5, 113.0, 122.3, 124.4, 125.4, 128.3, 128.7, 128.8, 128.9, 133.3, 139.7, 144.4, 164.4, 179.6, 188.4; IR (ATR KBr cell, cm−1) 732, 1491, 1614, 1722, 2829, 1241, 3284, 3425; LC-MS calcd m/z: 608 found 609 [(M + 1)]+. Anal. calcd for C25H24Br2N2O2S2: C, 49.35; H, 3.98; N, 4.60; C, 49.30; H, 3.96; N, 4.54.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(4-bromophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4lc. Off white solid; isolated yield 0.344 g (93%); M. Pt. 190–192 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.65–1.71 (m, 2H), 1.74–1.84 (m, 2H), 2.19 (s, 3H), 2.26 (s, 3H), 2.35–2.36 (m, 2H), 3.65–3.72 (m, 1H), 3.92–3.98 (m, 1H), 5.61 (s, 1H), 6.90 (d, J = 1.8 Hz, 1H), 7.10 (dd, J = 1.8 Hz, 7.96 Hz, 1H), 7.23 (d, J = 8.04 Hz, 1H), 7.35 (d, J = 8.48 Hz, 2H), 7.51 (d, J = 1.6 Hz, 2H), 10.65 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.5, 16.7, 27.5, 30.2, 47.6, 50.7, 67.6, 72.7, 73.2, 111.8, 112.9, 120.1, 122.2, 124.1, 125.3, 129.0, 130.5, 131.8, 140.4, 144.3, 164.2, 179.8, 188.6; IR (ATR KBr cell, cm−1) 760, 1126, 1348, 1490, 1708, 2878, 3380; LC-MS calcd m/z: 608 found 609 [(M + 1)]+. Anal. calcd for C25H24Br2N2O2S2: C, 49.35; H, 3.98; N, 4.60; found: C, 49.30; H, 3.94; N, 4.58.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(3-bromo-4-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4sc. Off white solid; isolated yield 0.328 g (91%); M. Pt. 150–152 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.68–1.72 (m, 2H), 1.76–1.81 (m, 2H), 2.19 (s, 3H), 2.27 (s, 3H), 2.32–2.38 (m, 2H), 3.69–3.76 (m, 2H), 3.95 (d, J = 11.6 Hz, 1H), 5.63 (s, 3H), 6.91 (d, J = 1.76 Hz, 1H), 7.09 (dd, J = 1.76 Hz, 7.96 Hz, 1H), 7.23 (t, J = 7.89 Hz, 1H), 7.33 (t, J = 8.68 Hz, 1H), 7.42–7.46 (m, 1H), 7.76 (dd, J = 2.08 Hz, 6.8 Hz, 1H), 10.70 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.6, 27.3, 29.9, 47.6, 50.1, 67.6, 72.6, 73.2, 112.0, 112.9, 117.1, 117.3, 122.3, 124.1, 125.3, 129.0, 133.3, 144.3, 164.1, 179.8, 185.6; IR (ATR KBr cell, cm−1) 748, 1128, 1348, 1494, 1608, 1710, 3328; LC-MS calcd m/z: 626 found 627 [(M + 1)]+. Anal. calcd for C25H23Br2FN2O2S2: C, 47.94; H, 3.70; N, 4.47: found: C, 47.90; H, 3.68; N, 4.43.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(5-bromothiophen-2-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4tc. Off white solid; isolated yield 0.345 g (94%); M. Pt. 130–132 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.67–1.78 (m, 2H), 1.79–1.95 (m, 1H), 2.24 (s, 3H), 2.32 (s, 3H), 2.35–2.46 (m, 2H), 3.78–3.83 (m, 2H), 3.91–3.97 (m, 1H), 5.66 (s, 1H), 6.87 (d, J = 3.64 Hz, 1H), 6.92 (s, 1H), 7.06–7.10 (m, 2H), 7.19 (d, J = 8 Hz, 1H), 10.65 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.4, 30.2, 46.7, 47.6, 68.5, 72.2, 73.3, 109.4, 111.7, 112.9, 122.4, 124.2, 124.9, 126.1, 129.2, 130.6, 144.2, 146.1, 164.8, 179.6, 188.1; IR (ATR KBr cell, cm−1) 761, 1384, 1606, 1700, 2456, 3071, 3309; LC-MS calcd m/z: 614 found 615 [(M + 1)]+. Anal. calcd for C23H22Br2N2O2S3: C, 44.96; H, 3.61; N, 4.56; found: C, 44.93; H, 3.56; N, 4.52.
2′-(3,3-Bis(methylthio)acryloyl)-6-bromo-1′-(5-bromopyridin-3-yl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4uc. Off white solid; isolated yield 0.34 g (92%); M. Pt. 194–196 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.79–1.80 (m, 2H), 1.81–1.84 (m, 2H), 2.21 (s, 3H), 2.29 (s, 3H), 2.38–2.45 (m, 2H), 3.74–3.79 (m, 2H), 4.08 (d, J = 11.36 Hz, 1H), 5.65 (s, 1H), 6.94 (s, 1H), 7.12 (dd, J = 1.36 Hz, 7.88 Hz, 1H), 7.25 (d, J = 8 Hz, 1H), 8.12 (s, 1H), 8.58 (s, 1H), 8.64 (s, 1H), 10.69 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δC: 14.6, 16.7, 27.4, 29.8, 47.7, 48.4, 67.2, 72.2, 73.2, 112.0, 112.9, 120.8, 122.4, 125.2, 129.0, 138.1, 138.9, 144.3, 148.6, 149.1, 164.1, 179.7, 188.4; IR (ATR KBr cell, cm−1) 709, 1480, 1722, 2827, 3248, 3423; LC-MS calcd m/z: 609 found 610 [(M + 1)]+. Anal. calcd for C24H23Br2N3O2S2: C, 47.30; H, 3.80; N, 6.90; found: C, 47.28; H, 3.76; N, 6.85.
General procedure for the synthesis of 5
A mixture of isatin 1 (1 mol), L-phenylalanine 2 (1 mol) was taken in methanol and heated for 15 min. This led to the formation of azomethine ylide which was treated with 1,1-bis(methylthio)-5-arylpenta-1,4-dien-3-one 3 and continued heating for further indicated time in Table 2. After completion of the reaction checked by TLC, the mixture was concentrated in vacuo. Column chromatography of the residue gave the pure product 5.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-6-bromo-4′-phenylspiro[indoline-3,2′-pyrrolidin]-2-one 5a. Pale yellow solid; isolated yield 0.398 g (86%); M. Pt. 130 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.13–2.22 (m, 6H), 2.68–2.73 (m, 2H), 3.58–3.67 (m, 3H), 3.92 (s, 1H), 5.48 (s, 1H), 6.86–6.92 (m, 2H), 6.99–7.07 (m, 3H), 7.11–7.39 (m, 9H), 10.60 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.4, 14.6, 16.5, 22.2, 52.5, 66.2, 67.6, 69.0, 112.1, 113.1, 121.7, 126.3, 127.1, 127.7, 128.5, 128.8, 129.0, 129.5, 139.6, 140.8, 143.8, 162.9, 189.5; IR (ATR KBr cell, cm−1) 740, 1520, 1600, 1726, 2967, 3140, 3240; LC-MS calcd m/z: 579 found 580 [(M + 1)]+. Anal. calcd for C29H27BrN2O2S2: C, 60.10; H, 4.70; N, 4.83; found: C, 60.05; H, 4.67; N, 4.76.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-4′-o-tolylspiro[indoline-3,2′-pyrrolidin]-2-one 5b. Pale yellow solid; isolated yield 0.311 g (80%); M. Pt. 122 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.11 (s, 3H), 2.19 (s, 3H), 2.54 (s, 3H), 2.58–2.64 (m, 1H), 2.70–2.76 (m, 2H), 3.08–3.10 (m, 1H), 3.69 (d, J = 10 Hz, 1H), 3.88–3.96 (m, 2H), 5.36 (s, 1H), 6.74 (d, J = 7.6 Hz, 1H), 6.85 (td, J = 0.8 Hz, 7.6 Hz, 1H), 7.06–7.24 (m, 10H), 7.49 (d, J = 7.6 Hz, 1H), 10.47 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.5, 14.8, 16.4, 19.8, 20.3, 48.5, 68.1, 69.0, 69.5, 109.2, 113.2, 121.8, 125.9, 126.2, 126.5, 126.7, 127.1, 128.4, 129.1, 129.2, 130.5, 137.4, 137.6, 139.8, 140.3, 142.1, 162.0, 182.3, 190.2; IR (ATR KBr cell, cm−1) 748, 1619, 1750, 2948, 3300; LC-MS calcd m/z: 514 found 515 [(M + 1)]+. Anal. calcd for C30H30N2O2S2: C, 70.01; H, 5.87; N, 5.44; found: C, 69.07; H, 5.80; N, 5.41.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-4′-(4-isopropylphenyl)spiro[indoline-3,2′-pyrrolidin]-2-one 5d. Pale yellow solid; isolated yield 0.315 g (85%); M. Pt. 172 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.09–1.21 (m, 6H), 2.11–2.22 (m, 6H), 2.63–2.74 (m, 2H), 2.83–2.94 (m, 2H), 3.51–3.56 (m, 1H), 3.62 (d, J = 10.8 Hz, 1H), 3.87–3.88 (m, 1H), 5.40 (s, 1H), 6.71 (d, J = 7.6 Hz, 1H), 6.82 (t, J = 7.2 Hz, 1H), 6.89 (d, J = 7.6 Hz, 1H), 7.08–7.22 (m, 9H), 7.31 (d, J = 8 Hz, 2H), 10.44 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.5, 16.4, 24.3, 24.4, 33.5, 52.4, 66.0, 69.3, 109.2, 113.1, 121.8, 125.8, 126.3, 127.0, 128.4, 128.6, 129.6, 142.1, 147.0, 142.1, 147.0, 189.3; IR (ATR KBr cell, cm−1) 780, 1168, 1600, 1720, 2897, 3300; LC-MS calcd m/z: 542 found 543 [(M + 1)]+. Anal. calcd for C32H34N2O2S2: C, 70.81; H, 6.31; N, 5.16; found: C, 70.79; H, 6.27; N, 5.13.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-6-bromo-4′-(3-methoxyphenyl)spiro[indoline-3,2′-pyrrolidin]-2-one 5g. Pale yellow solid; isolated yield 0.377 g (87%); M. Pt. 196 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.19 (s, 3H), 2.22 (s, 3H), 2.59–2.68 (m, 1H), 2.75 (dd, J = 3.2 Hz, 14 Hz, 1H), 3.54 (t, J = 10.4 Hz, 1H), 3.63 (d, J = 10.8 Hz, 1H), 3.76 (s, 3H), 3.86–3.87 (m, 1H), 5.49 (s, 1H), 6.80–6.87 (m, 3H), 6.93 (s, 1H), 6.97 (d, J = 7.6 Hz, 1H), 7.01 (dd, J = 1.6 Hz, 7.6 Hz, 1H), 7.09–7.16 (m, 3H), 7.21–7.30 (m, 3H), 10.59 (s, 1H). 13C NMR (75 MHz, DMSO-d6) δC: 14.6, 16.5, 52.6, 55.4, 66.1, 67.6, 69.1, 112.0, 112.1, 113.1, 114.9, 120.8, 121.6, 124.4, 126.3, 127.6, 128.4, 128.8, 129.5, 129.8, 130.0, 130.3, 130.4, 139.8, 142.6, 143.8, 159.8, 162.7, 182.0, 189.6; IR (ATR KBr cell, cm−1) 765, 1299, 1610, 1740, 2696, 3280; LC-MS calcd m/z: 609 found 610 [(M + 1)]+. Anal. calcd for C30H29BrN2O3S2: C, 59.11; H, 4.80; N, 4.60; found: C, 59.05; H, 4.73; N, 4.57.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-4′-(4-bromophenyl)spiro[indoline-3,2′-pyrrolidin]-2-one 5l. Pale yellow solid; isolated yield 0.299 g (85%); M. Pt. 118 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.14 (s, 3H), 2.18 (s, 3H), 2.70 (d, J = 6 Hz, 2H), 3.54–3.62 (m, 2H), 3.85–3.88 (m, 1H), 5.39 (s, 1H), 6.72 (d, J = 7.6 Hz, 1H), 6.81 (td, J = 3.2 Hz, 6.8 Hz, 1H), 6.91 (d, J = 8 Hz, 1H), 7.09–7.23 (m, 6H), 7.34 (J = 8.4 Hz, 2H), 7.53 (d, J = 8.4 Hz, 2H), 10.46 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.6, 16.4, 52.3, 66.1, 67.8, 69.3, 109.3, 113.0, 120.0, 121.8, 125.8, 126.3, 128.4, 129.1, 129.6, 130.4, 130.6, 131.0, 131.8, 139.6, 140.7, 142.0, 162.4, 182.0, 189.5: IR (ATR KBr cell, cm−1) 720, 1019, 1336, 1490, 1640, 1748, 3220, 3380; LC-MS calcd m/z: 579 found 580 [(M + 1)]+. Anal. calcd for C29H27BrN2O2S2: C, 60.10; H, 4.70; N, 4.83; found: C, 60.05; H, 4.67; N, 4.80.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-4′-(furan-2-yl)spiro[indoline-3,2′-pyrrolidin]-2-one 5v. Pale yellow solid; isolated yield 0.318 g (78%); M. Pt. 118 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.19 (s, 3H), 2.21 (s, 3H), 2.74–2.79 (m, 1H), 2.89–2.97 (m, 2H), 3.65–3.82 (m, 1H), 5.49 (s, 1H), 6.25–6.26 (m, 1H), 6.38–6.39 (m, 1H), 6.70 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 4 Hz, 2H), 7.07–7.27 (m, 6H), 7.58 (s, 1H), 10.43 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.6, 16.5, 45.7, 63.7, 64.3, 69.0, 106.8, 109.2, 110.9, 112.7, 121.8, 122.0, 125.7, 126.4, 128.5, 129.1, 129.6, 129.8, 139.4, 142.0, 154.3, 162.7, 181.7, 189.2; IR (ATR KBr cell, cm−1) 704, 1081, 1280, 1490, 1649, 1720, 2916, 3100; LC-MS calcd m/z: 490 found 491 [(M + 1)]+. Anal. calcd for C27H26N2O3S2: C, 66.10; H, 5.34; N, 5.71; found: C, 66.04; H, 5.30; N, 5.68.
5′-Benzyl-3′-(3,3-bis(methylthio)acryloyl)-5-methoxy-4′-(naphthalen-1-yl)spiro[indoline-3,2′-pyrrolidin]-2-one 5w. Pale yellow solid; isolated yield 0.313 g (81%); M. Pt. 118 °C; 1H NMR (400 MHz, DMSO-d6) δH: 2.07 (s, 3H), 2.17 (s, 3H), 2.77–2.82 (m, 1H), 3.12 (s, 1H), 3.68–3.74 (m, 4H), 3.83–3.88 (m, 1H), 4.06 (s, 1H), 5.42 (s, 1H), 6.68–6.79 (m, 4H), 7.01–7.17 (m, 6H), 7.48–8.21 (m, 6H), 10.32 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 13.0, 15.1, 54.9, 66.4, 67.7, 69.4, 105.2, 109.6, 110.9, 112.0, 124.8, 125.0, 125.2, 126.1, 127.0, 127.9, 128.1148.8, 154.2, 157.6, 163.0, 180.2, 188.3; IR (ATR KBr cell, cm−1) 738, 1136, 1430, 1646, 1749, 2860, 3320; LC-MS calcd m/z: 580 found 581 [(M + 1)]+. Anal. calcd for C34H32N2O3S2: C, 70.32; H, 5.55; N, 4.82; found: C, 70.28; H, 5.50; N, 4.80.
2′-(1H-Benzo[d]imidazol-2-yl)-1′-(4-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 8a. Off white solid; isolated yield 0.073 g (78%); M. Pt. 138 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.81–1.96 (m, 2H), 2.42–2.45 (m, 1H), 2.70–2.75 (m, 1H), 3.67 (s, 3H), 3.89–3.95 (m, 1H), 4.01–4.09 (m, 1H), 4.38 (d, J = 12.4 Hz, 1H), 6.59 (d, J = 7.6 Hz, 1H), 6.77 (td, J = 0.8 Hz, 7.6 Hz, 1H), 6.85 (d, J = 8.8 Hz, 2H), 6.95–7.01 (m, 3H), 7.15–7.17 (m, 1H), 7.29 (d, J = 7.2 Hz, 1H), 7.36–7.39 (m, 3H), 10.29 (s, 1H), 11.84 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 27.3, 30.4, 48.0, 53.0, 55.4, 57.3, 72.4, 74.4, 109.9, 111.2, 114.5, 118.7, 120.9, 121.2, 121.9, 126.2, 127.3, 129.0, 129.3, 132.3, 134.3, 142.6, 143.2, 151.5, 158.5, 169.0, 179.8; IR (ATR KBr cell, cm−1) 831, 1125, 1428, 1404, 1625, 2981, 3481; LC-MS calcd m/z: 450 found 451 [(M + 1)]+. Anal. calcd for C28H26N4O2: C, 74.65; H, 5.82; N, 12.44; found: C, 74.59; H, 5.78; N, 12.40.
2′-(1H-Benzo[d]imidazol-2-yl)-6-bromo-1′-(3-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 8b. Off white solid; isolated yield 0.076 g (80%); M. Pt. 144 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.74–1.81 (m, 2H), 1.92–1.96 (m, 2H), 2.20–2.26 (m, 1H), 2.68–2.71 (m, 1H), 3.48–3.55 (m, 1H), 3.69 (m, 1H), 3.94–3.97 (m, 1H), 4.11 (t, J = 9.6 Hz, 1H), 4.43 (d, J = 12 Hz, 1H), 6.73–6.76 (m, 1H), 6.92–7.03 (m, 5H), 7.18–7.29 (m, 3H), 7.39–7.42 (m, 1H), 10.48 (s, 1H), 11.94 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 27.3, 30.3, 48.0, 53.8, 55.4, 56.9, 72.2, 74.2, 111.3, 112.3, 112.8, 114.2, 120.1, 121.4, 122.0, 122.1, 123.6, 125.5, 130.1, 134.3, 142.0, 143.2, 144.4, 151.3, 159.8, 179.6; IR (ATR KBr cell, cm−1) 752, 1134, 1332, 1625, 2883, 3441; LC-MS calcd m/z: 528 found 529 [(M + 1)]+. Anal. calcd for C28H25BrN4O2: C, 63.52; H, 4.76; N, 10.58; found: C, 63.48; H, 4.73; N, 10.53.
2′-(1H-Benzo[d]imidazol-2-yl)-1′-p-tolyl-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 8c. Off white solid; isolated yield 0.082 g (88%); M. Pt. 148 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.72–1.80 (m, 2H), 1.92–1.97 (m, 2H), 2.20 (s, 3H), 2.25–2.27 (m, 1H), 2.41–2.45 (m, 1H), 2.72–2.74 (m, 1H), 3.92–3.95 (m, 1H), 4.06–4.11 (m, 1H), 4.42 (d, J = 12.4 Hz, 1H), 6.60 (d, J = 7.2 Hz, 1H), 6.77 (t, J = 7.6 Hz, 1H), 6.95–7.01 (m, 3H), 7.08 (d, J = 8 Hz, 2H), 7.12–7.17 (m, 1H), 7.27–7.35 (m, 4H), 10.30 (s, 1H), 11.85 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 21.0, 27.3, 30.4, 48.0, 53.4, 57.2, 72.4, 74.4, 109.9, 120.9, 121.9, 126.2, 127.4, 129.3, 129.6, 136.3, 137.5, 142.6, 151.5, 179.8; IR (ATR KBr cell, cm−1) 752, 1134, 1332, 1625, 2883, 3441; LC-MS calcd m/z: 434 found 435 [(M + 1)]+. Anal. calcd for C28H26N4O: C, 77.39; H, 6.03; N, 12.89; found: C, 77.33; H, 6.00; N, 12.85.
2′-(2-Amino-6-methoxypyrimidin-4-yl)-1′-(4-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 10a. Off white solid; isolated yield 0.081 g (85%); M. Pt. 118 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.43–1.74 (m, 2H), 1.80–1.92 (m, 2H), 2.33–2.53 (m, 2H), 3.55 (s, 3H), 3.68–3.81 (m, 5H), 4.00 (d, J = 12 Hz, 1H), 5.59 (s, 1H), 6.12 (s, 1H), 6.66 (d, J = 7.6 Hz, 1H), 6.86 (d, J = 8.4 Hz, 3H), 7.08 (td, J = 0.8 Hz, 7.6 Hz, 1H), 7.31 (d, J = 8.8 Hz, 2H), 7.38 (d, J = 7.6 Hz, 1H), 10.10 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 27.8, 30.7, 47.6, 51.4, 53.0, 55.4, 62.7, 73.1, 74.9, 95.1, 109.8, 114.4, 120.7, 126.2, 127.5, 129.0, 132.6, 142.8, 158.4, 162.8, 167.1, 170.0, 180.0; IR (ATR KBr cell, cm−1) 1140, 1750, 2380, 3328; LC-MS calcd m/z: 457 found 458 [(M + 1)]+. Anal. calcd for C26H27N5O3: C, 68.25; H, 5.95; N, 15.31; found: C, 68.21; H, 5.90; N, 15.29.
2′-(2-Amino-6-ethoxypyrimidin-4-yl)-1′-(4-methoxyphenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 10b. Off white solid; isolated yield 0.078 g (80%); M. Pt. 108 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.09–1.28 (m, 3H), 1.69–1.98 (m, 4H), 2.19–2.36 (m, 2H), 3.66–3.79 (m, 6H), 3.97–4.00 (m, 2H), 5.98 (s, 1H), 6.00 (s, 1H), 6.70–6.86 (m, 4H), 7.03–7.07 (m, 1H), 7.29–7.34 (m, 3H), 10.05 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.7, 27.7, 30.6, 47.6, 51.2, 55.4, 61.2, 62.4, 73.2, 74.9, 95.1, 109.8, 114.4, 120.6, 127.6, 129.0, 132.7, 143.0, 158.4, 162.8, 167.1, 170.0, 180.9; IR (ATR KBr cell, cm−1) 1125, 1748, 2520, 3152, 3420; LC-MS calcd m/z: 471 found 472 [(M + 1)]+. Anal. calcd for C27H27N5O3: C, 68.77; H, 6.20; N, 14.85; found: C, 68.73; H, 6.15; N, 14.83.
2′-(2-Amino-6-methoxypyrimidin-4yl)-1′-(4-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 10c. Off white solid; isolated yield 0.076 g (80%); M. Pt. 128 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.70–1.73 (m, 2H), 1.83–1.89 (m, 2H), 2.36–2.40 (m, 1H), 2.48–2.53 (m, 1H), 3.56 (s, 3H), 3.74–3.78 (m, 1H), 3.85–3.91 (m, 1H), 4.01 (d, J = 12.4 Hz, 1H), 5.60 (s, 1H), 6.15 (s, 2H), 6.67 (d, J = 7.6 Hz, 1H), 6.87 (td, J = 1.2 Hz, 7.6 Hz, 1H), 7.06–7.15 (m, 3H), 7.38 (d, J = 7.6 Hz, 1H), 7.42–7.46 (m, 2H), 10.14 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 27.7, 30.6, 47.6, 51.3, 53.0, 62.8, 73.0, 74.8, 95.0, 109.8, 115.6, 115.8, 120.7, 126.1, 127.6, 129.1, 129.8, 129.9, 137.0, 142.8, 162.8, 166.8, 170.1, 179.9; IR (ATR KBr cell, cm−1) 1176, 1740, 2360, 3340; LC-MS calcd m/z: 445 found 446 [(M + 1)]+. Anal. calcd for C25H24FN5O2: C, 67.40; H, 5.43; N, 15.72; found: C, 67.33; H, 5.40; N, 15.68.
2′-(2-Amino-6-ethoxypyrimidin-4-yl)-1′-(4-fluorophenyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 10d. Off white solid; isolated yield 0.076 g (78%); M. Pt. 116 °C; 1H NMR (400 MHz, DMSO-d6) δH: 1.15 (t, J = 6.8 Hz, 3H), 1.67–1.75 (m, 2H), 1.80–1.91 (m, 2H), 2.35–2.40 (m, 1H), 2.46–2.52 (m, 1H), 3.72–3.80 (m, 1H), 3.86–3.91 (m, 1H), 3.96–4.04 (m, 1H), 5.59 (s, 1H), 6.10 (s, 1H), 6.67 (d, J = 7.6 Hz, 1H), 6.87 (td, J = 0.8 Hz, 7.6 Hz, 1H), 7.06–7.15 (m, 3H), 7.38 (d, J = 7.2 Hz, 1H), 7.43–7.46 (m, 2H), 10.13 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δC: 14.7, 27.7, 30.6, 47.6, 51.2, 61.2, 62.7, 73.1, 74.8, 95.1, 109.8, 115.6, 115.8, 120.7, 126.1, 127.6, 129.0, 129.8, 129.9, 137.1, 142.8, 159.8, 162.8, 163.0, 166.8, 169.7, 179.9; IR (ATR KBr cell, cm−1) 856, 1180, 1700, 2490, 3390; LC-MS calcd m/z: 459 found 460 [(M + 1)]+. Anal. calcd for C26H26FN5O2: C, 67.96; H, 5.70; N, 15.24; found: C, 67.93; H, 5.65; N, 15.18.
Acknowledgements
SS thank DST and UGC-MRP, New Delhi, for financial assistance. We thank DST-IRHPA for funding towards higher resolution NMR spectrometer. PD thanks the UGC, New Delhi for the fellowship under UGC-BSR Meritorious. We thank Dr P. Thilagar, Department of IPC, IISc Bangalore for analyzing the single crystal X-ray data.
Notes and references
-
(a) A. D. Melhado, W. E. Brenzovitch, A. D. Lackner and F. D. A. Toste, J. Am. Chem. Soc., 2010, 132, 8885–8887 CrossRef CAS PubMed;
(b) G. L. Adams, P. J. Carroll and A. B. Smith, J. Am. Chem. Soc., 2013, 135, 519–528 CrossRef CAS PubMed;
(c) S. A. Snyder, S. P. Breazzano, A. G. Ross, Y. Lin and A. L. Zografos, J. Am. Chem. Soc., 2009, 131, 1753–1765 CrossRef CAS PubMed;
(d) R. A. Yoder and J. N. Johnston, Chem. Rev., 2005, 105, 4730–4756 CrossRef CAS PubMed;
(e) J. K. Sutherland, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon Press, Elmsford, NY, 1991, vol. 1, pp. 341–377 Search PubMed.
- For recent reviews:
(a) M. A. Borad, M. N. Bhoi, N. P. Prajapati and H. D. Patel, Synth. Commun., 2014, 44, 897–922 CrossRef CAS;
(b) N. Lashgari and G. M. Ziarani, ARKIVOC, 2012, 277–320 CrossRef CAS;
(c) M. M. M. Santos, Tetrahedron, 2014, 70, 9735–9757 CrossRef CAS PubMed.
-
(a) S. T. Hilton, T. C. T. Ho, G. Pljevaljcic and K. Jones, Org. Lett., 2000, 2, 2639–2641 CrossRef CAS;
(b) F. Yu, R. Huang, H. Ni, J. Fan, S. Yan and J. Lin, Green Chem., 2013, 15, 453–462 RSC.
-
(a) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219 CrossRef CAS PubMed;
(b) R. M. Williams and R. J. Cox, Acc. Chem. Res., 2003, 36, 127–139 CrossRef CAS PubMed.
-
(a) A. Jossang, P. Jossang, H. A. Hadi, T. Sevenet and B. Bodo, J. Org. Chem., 1991, 56, 6527–6530 CrossRef CAS;
(b) U. K. Syam Kumar, H. Ila and H. Junjappa, Org. Lett., 2001, 3, 4193–4196 CrossRef CAS PubMed.
- C. B. Cui, H. Kakeya, G. Okada and R. Onose, J. Antibiot., 1996, 49, 527–533 CrossRef CAS.
- M. N. G. James and G. J. B. Williams, Can. J. Chem., 1972, 50, 2407–2412 CrossRef CAS.
- R. C. Elderfield and R. E. Gilman, Phytochemistry, 1972, 11, 339–343 CrossRef CAS.
- K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS PubMed.
-
(a) T. Okita and M. Isobe, Tetrahedron, 1994, 50, 11143–11152 CrossRef CAS;
(b) M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892–898 CrossRef CAS;
(c) A. Thangamani, Eur. J. Med. Chem., 2010, 45, 6120–6126 CrossRef CAS PubMed.
-
(a) R. Ranjithkumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, J. Med. Chem., 2008, 51, 5731–5735 CrossRef PubMed;
(b) R. Ranjithkumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411–422 CrossRef PubMed.
- S. V. Karthikeyan, B. Devi Bala, V. P. Alex Raja, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 350–353 CrossRef CAS PubMed.
- A. Dhandia, S. Kumar and P. Soni, Eur. Chem. Bull., 2013, 2, 1004–1008 Search PubMed.
-
(a) J. W. Daly, T. W. Spande, N. Whittaker, R. J. Highet, D. Feigl, N. Noshimori, T. Tokuyama and C. W. Meyers, J. Nat. Prod., 1986, 49, 265–280 CrossRef CAS;
(b) H. Waldmann, Synlett, 1995, 133 CrossRef CAS PubMed;
(c) A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 91–100 CrossRef CAS PubMed;
(d) A. A. Raj, R. Raghunathan, M. R. Sridevikumari and N. Raman, Bioorg. Med. Chem., 2003, 11, 407–419 CrossRef CAS.
-
(a) F. Zhou, Y. L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS PubMed;
(b) G. S. Singh and A. Y. Desta, Chem. Rev., 2012, 112, 6104–6105 CrossRef CAS PubMed;
(c) S. Morteza, Chem. Rev., 2012, 112, 3508–3549 CrossRef PubMed;
(d) A. Millemaggi and R. J. K. Taylor, Eur. J. Org. Chem., 2010, 4527–4547 CrossRef CAS PubMed.
- K. B. G. Torssell, Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis, Novel strategies in synthesis, VCH, Weinheim, 1988 Search PubMed.
-
(a) D. Fokas, W. J. Ryan, D. S. Casebier and D. L. Coffen, Tetrahedron Lett., 1998, 39, 2235–2238 CrossRef CAS;
(b) R. Ranjithkumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Tetrahedron, 2008, 64, 2962–2971 CrossRef PubMed;
(c) M. Bakthadoss, D. Kannan and G. Sivakumar, Synthesis, 2012, 793–799 CrossRef CAS PubMed;
(d) R. D. R. S. Manian, J. Jayashankaran and R. Raghunathan, Tetrahedron, 2006, 62, 12357–12362 CrossRef CAS PubMed.
-
(a) J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2012, 53, 854–858 CrossRef CAS PubMed;
(b) V. Rajkumar, N. A. Aslam, C. Reddy and S. A Babu, Synlett, 2012, 549–556 CAS;
(c) A. R. Suresh Babu and R. Raghunathan, Tetrahedron Lett., 2007, 48, 305–308 CrossRef PubMed;
(d) A. R. Suresh Babu and R. Raghunathan, Tetrahedron Lett., 2007, 48, 6809–6813 CrossRef PubMed.
-
(a) R. Jain, K. Sharma and D. Kumar, Tetrahedron Lett., 2012, 53, 1993–1997 CrossRef CAS PubMed;
(b) A. Dandia, A. K. Jain and D. S. Bhati, Tetrahedron Lett., 2011, 52, 5333–5337 CrossRef CAS PubMed;
(c) H. Liu, G. Dou and D. Shi, J. Comb. Chem., 2010, 12, 633–637 CrossRef CAS PubMed;
(d) J. Li, J. Wang, Z. Xu and S. Zhu, J. Comb. Chem., 2014, 16, 506–512 CAS;
(e) N. V. Lakshmi, P. Thirumurugan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 1064–1068 CrossRef CAS PubMed.
-
(a) A. R. Suresh Babu, R. Raghunathan, G. Gayatri and G. N. Sastry, J. Heterocycl. Chem., 2006, 43, 1467–1472 CrossRef PubMed;
(b) A. A. Watson, G. W. J. Fleet, N. Asano, R. J. Molyneux and R. J. Nash, Phytochemistry, 2001, 56, 265–295 CrossRef CAS;
(c) D. O'Hagan, Nat. Prod. Rep., 1997, 14, 637–651 RSC;
(d) S. Horri, H. Fukase, T. Matsuo, Y. Kameda, N. Asano and K. Matsui, J. Med. Chem., 1986, 29, 1038–1046 CrossRef;
(e) M. A. Spearman, J. C. Jamieson and J. A. Wright, Exp. Cell Res., 1987, 168, 116–126 CrossRef CAS;
(f) A. Karpas, G. W. J. Fleet, R. A. Dwek, S. Petursson, S. K. Mamgoong, N. G. Ramsden, G. S. Jacob and T. W. Rademacher, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 9229–9233 CrossRef CAS;
(g) J. R. Liddell, Nat. Prod. Rep., 1998, 15, 363–370 RSC;
(h) J. P. Michael, Nat. Prod. Rep., 1997, 14, 619–636 RSC.
-
(a) K. Revathy and A. Lalitha, RSC Adv., 2014, 4, 279–285 RSC;
(b) R. Murugan, R. Raghunathan and S. S. Narayanan, Synth. Commun., 2010, 40, 3135–3151 CrossRef CAS;
(c) R. Rajesh and R. Raghunathan, Tetrahedron Lett., 2010, 51, 5845–5848 CrossRef CAS PubMed.
- P. Dhanalakshmi and S. Sivakumar, RSC Adv., 2014, 4, 29493–29501 RSC.
-
(a) Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC;
(b) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC;
(c) A. Nagaraju, B. J. Ramulu, G. Shukla, A. Srivastava, G. K. Verma, K. Raghuvanshi and M. S. Singh, Green Chem., 2015, 17, 950–958 RSC;
(d) S. Vidyacharan, A. H. Shinde, B. Satpathi and D. S. Sharada, Green Chem., 2014, 16, 1168–1175 RSC;
(e) M. Li, A. Taheri, M. Liu, S. Sun and Y. Gu, Adv. Synth. Catal., 2014, 356, 537–556 CrossRef CAS PubMed.
-
(a) B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef CAS PubMed;
(b) A. Padwa, Chem. Soc. Rev., 2009, 38, 3072–3081 RSC;
(c) A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed;
(d) D. M. D'Souza and T. J. J. Muller, Chem. Soc. Rev., 2007, 36, 1095–1108 RSC;
(e) L. F. Tietze and F. Haunert, in Stimulating Concepts in Chemistry, ed. F. Vogtle, J. F. Stoddart and M. Shibasaki, Wiley-VCH, Weinheim, 2000, pp. 39–64 Search PubMed;
(f) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484–491 RSC;
(g) V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546–1557 RSC.
-
(a) L. Weber, Curr. Med. Chem., 2002, 9, 2085–2093 CrossRef CAS;
(b) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51–80 CrossRef CAS.
-
(a) C. J. O'Connor, H. S. J. Beckmann and D. R. Spring, Chem. Soc. Rev., 2012, 41, 4444–4456 RSC;
(b) For a review of the use of multicomponent reactions in diversity-oriented synthesis, see:;
(c) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef CAS PubMed.
- For selected examples see:
(a) W. Singh, A. K. Gupta, H. Ila and H. Junjappa, Synthesis, 1984, 516–518 CrossRef;
(b) A. Thuiller and J. Vialle, Bull. Soc. Chim. Fr., 1962, 2182 Search PubMed;
(c) B. Myrboh, C. V. Ashokan, H. Ila and H. Junjappa, Synthesis, 1984, 50–51 CrossRef CAS;
(d) H. S. P. Rao and S. Sivakumar, Beilstein J. Org. Chem., 2007, 3, 31 CrossRef PubMed;
(e) Y. L. Zhao, L. Chen, S. C. Yang, C. Tian and Q. Liu, J. Org. Chem., 2009, 74, 5622–5625 CrossRef CAS PubMed;
(f) Y. Ma, M. Wang, D. Li, B. Bekturhun, J. Liu and Q. Liu, J. Org. Chem., 2009, 74, 3116–3121 CrossRef CAS PubMed;
(g) J. Tan, X. Xu, Y. Li and Q. Liu, Angew. Chem., Int. Ed., 2009, 48, 2868–2872 CrossRef CAS PubMed;
(h) H. Wang, Y. L. Zhao, C. Q. Ren, A. Diallo and Q. Liu, Chem. Commun., 2011, 47, 12316–12318 RSC;
(i) Y. Li, X. Xu, J. Tan, C. Xia, D. Zhang and Q. Liu, J. Am. Chem. Soc., 2011, 133, 1775–1777 CrossRef CAS PubMed.
- ESI.†.
-
(a) S. Pandey, S. N. Suryawanshi, S. Gupta and V. M. L. Srivastava, Eur. J. Med, Chem., 2005, 40, 751–756 CrossRef CAS PubMed;
(b) S. Kumar, A. Tiwari, S. N. Suryawanshi, M. Mittal, P. Vishwakarma and S. Gupta, Bioorg. Med. Chem., 2012, 22, 6728–6730 CrossRef CAS PubMed.
- P. Dhanalakshmi, S. Thimmarayaperumal and S. Sivakumar, RSC Adv., 2014, 4, 12028–12036 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 988416–988419 and 1040486. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra05123a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 



















