Radical copolymerisation of chlorotrifluoroethylene with isobutyl vinyl ether initiated by the persistent perfluoro-3-ethyl-2,4-dimethyl-3-pentyl radical†
Abstract
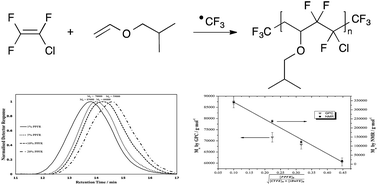
Results of the radical copolymerisation of chlorotrifluoroethylene (CTFE) with isobutyl vinyl ether (iBuVE) initiated by ˙CF3 radicals generated by β-scission of perfluoro-3-ethyl-2,4-dimethyl-3-pentyl radical (PPFR) at 90 °C in a batch reactor are reported. 19F NMR spectroscopy enabled the assessment of the molecular weights of the poly(CTFE-alt-iBuVE) copolymer by end-group analysis. It was found that, at low initiator concentrations (≤10 mol%), the ˙CF3 radicals preferably attack the vinyl ether monomer to initiate chain propagation and produce alternating poly(CTFE-alt-iBuVE) copolymers. At initiator ratios of 20 mol%, 19F NMR signals in the CF3 region other than the expected CH2–CF3 are observed and are attributed to ˙CF3 addition patterns due to kinetic effects brought on by monomer solubility. The molecular weights for the copolymer produced from 1%, 5%, and 10% PPFR were found to be 340 000, 237 000 and 122 000 g mol−1, respectively. The copolymer produced from 20% PPFR was oligomeric in nature with a molecular weight of 18 000 g mol−1.

 Please wait while we load your content...
Please wait while we load your content...