Hydrogen evolution inhibition by l-serine at the negative electrode of a lead–acid battery
Abstract
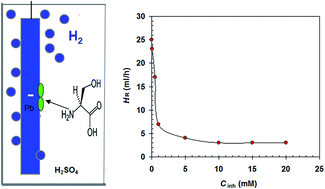
The inhibition effect of L-serine on the hydrogen evolution at the negative electrode of a lead–acid battery (Pb) in 5.0 M H2SO4 has been studied by hydrogen evolution and electrochemical methods. The surface of Pb is analyzed with a scanning electron microscope (SEM) and energy-dispersive X-ray (EDX). The results demonstrate that L-serine is an adequate inhibitor, retarding the hydrogen evolution reaction. Polarization curves denote that L-serine performs as the cathodic inhibitor. The inhibition efficiency increases with an increase in L-serine concentration but decreases with an upturn in temperature. The most efficient inhibitor concentration is 10 mM. Adsorption of L-serine on the Pb surface is unprompted and complies with Langmuir's isotherm. L-serine induces an energy barrier for the hydrogen evolution reaction and this barrier increases with increasing L-serine concentration.

 Please wait while we load your content...
Please wait while we load your content...