Rheological behavior of hydrophobically modified polysulfobetaine methacrylate aqueous solution
Miao Du*,
Yanjie Ma,
Heng Su,
Xiang Wang and
Qiang Zheng
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: dumiao@zju.edu.cn
First published on 2nd April 2015
Abstract
Polysulfobetaine methacrylate (PSBMA) that was hydrophobically modified with methacrylic acid 2,3-epoxypropyl ester (GMA) was synthesized via the micellar copolymerization method. Basic properties of the obtained copolymer were investigated via fluorescence, transmission electron microscopy (TEM), dynamic light scattering (DLS), zeta-potential analyzer, etc. The rheological properties of polymer (or copolymer) solutions were determined as a function of shear rate, temperature, NaCl concentration, etc. Hydrophobically modified PSBMA were capable of forming micelles even at extremely low concentrations (0.001 g L−1), and the micelle sizes increased upon increasing concentration. Hydrophobic interactions were observed within the system and were enhanced with increasing copolymer concentration. Both PSBMA with and without hydrophobic modification exhibited negative zeta-potential and increased with increasing polymer concentration. The viscosity of the P(SBMA–GMA) aqueous solution showed features that are different from those of typical polymer solutions. In addition, the rheological behavior of hydrophobically modified PSBMA aqueous solution had an unusual relationship with temperature because of hydrophobic association and epoxy groups. The viscosity of hydrophobically modified PSBMA solution was sensitive to the added salt concentration, i.e., the viscosity initially decreased and subsequently increased upon the addition of salt.
1 Introduction
Recently, studies have focused on polyzwitterionic compounds, including poly[2-methacryloyloxethyl phosphorylcholine (MPC)],1–3 polycarboxybetaine methacrylate (CBMA),4 and polysulfobetaine methacrylate (SBMA),5 because of their ultra-low-fouling property. Surfaces that are coated with these polymers allow for a nonspecific protein adsorption of up to 5 ng cm−2, which can effectively inhibit platelet adhesion for blood compatibility.6 The ultra-low-fouling property of polyzwitterionic materials is caused by the high hydration around the opposing charges and the high energetics required to remove that hydration layer.7 Therefore, polyzwitterionic compounds were reported to have considerable potential in many applications, such as medical implants,8 marine coatings,9 and biosensors.10In recent years, despite the excellent antifouling property of polyzwitterionic compounds, many scholars focused on solution properties, both theoretically11–15 and experimentally.16,17 The unique properties of polymeric betaines are strongly related to the opposite charge interactions in aqueous solutions.18,19 The states of polyzwitterionic chain can be varied by adjusting the temperature,20–22 pH value,23,24 salt concentration and so on.22,25 Dobry and Rubinstein developed the Flory theory of polyampholyte, and they proposed a two-parameter theoretical model that can forecast and explain several polyampholyte properties precisely.26
Hydrophobically associating polymers contain a small proportion of hydrophobic groups that are incorporated in a hydrophilic polymer chain (usually as pendant side chains or terminal groups). In aqueous solution, beyond a specific polymer concentration, a transitory three-dimensional network of polymer chains is formed as a result of the intermolecular interactions of the hydrophobic group.27 These compounds are used in many applications, such as water-borne coatings, paints, cosmetics, and enhanced oil recovery.28–30 From a technological perspective, the rheological behavior of these associative polymers is a highly significant feature. Hill and his coworkers31,32 investigated the system of hydrophobically associating polyacrylamides. They proposed a mechanism of this synthetic method and stated that the microstructure can be adjusted by varying the hydrophobe/surfactant ratio, i.e., the number of hydrophobes per micelle.31 Meanwhile, several researchers have found that these polymers have unique rheological behaviors.33–35 However, to our best knowledge, few studies have focused on the rheological behavior of polyzwitterionic compounds that are modified by hydrophobic monomers.36,37 With more and more application of polyzwitterionic compounds in a solution mode, it is necessary to explore the corresponding rheological behavior.
In the present paper, hydrophobic monomers are introduced into the polyzwitterionic chain backbones via the micellar copolymerization method.31 The aggregation properties of associative polymers are characterized via fluorescence measurement, and the morphology of these hydrophobically associating polymers are observed via transmission electron microscopy (TEM). Subsequently, the effects of concentration (c), salt addition, and temperature on the rheological behavior of hydrophobically modified polyzwitterionic compound aqueous solution are also discussed.
2 Experimental section
2.1 Materials
N-(3-Sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betain (SBMA) was purchased from Sigma-Aldrich. Methacrylic acid 2,3-epoxypropyl ester (GMA) was purchased from Aladdin. Tween 20 was purchased from Wenzhou Qingming Chemical Co., Ltd. 2,2′-Azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (VA-044) was purchased from J&K Scientific Ltd. The water used for all experiments was ultrapure and supplied by a water purification system. All of the other reagents were used without further purification.2.2 Synthesis of SBMA–GMA copolymer
SBMA–GMA copolymer [P(SBMA–GMA)] was synthesized via micellar copolymerization31,32 in 1 mol L−1 NaCl aqueous solution, and VA-044 was used as the initiator. The reaction vessel was a four-necked flask equipped with a condenser tube, mechanical stirrer, thermometer, and nitrogen inlet/outlet. Tween 20, which functions as an emulsifier, and GMA were first dissolved in 1 mol L−1 NaCl solution (40 mL) and were then transferred into the flask with stirring. After 0.5 h, SBMA dissolved in 1 mol L−1 NaCl solution (50 mL) was poured into the flask. The mass ratio of SBMA to GMA is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was stirred in an inert nitrogen atmosphere and heated at 50 °C in an oil bath. After 0.5 h, VA-044 dissolved in 1 mol L−1 NaCl solution (10 mL) was injected using a syringe. The reaction proceeded for 7 h. After cooling the final reaction mixture, the copolymer was precipitated by dropwise addition into excess ethanol, and subsequent dissolution–precipitation for three times. The final precipitate obtained was put in a Petri dish and then was stored in a refrigerator at −30 °C for about 24 h. After completely frozen, the sample was then transferred it into a vacuum freeze drier (FD-1-50, Beijing Boyikang Laboratory Instruments Co., Ltd) at −51 °C, 15 Pa for 12 h. At last, the dry product was obtained. In contrast with the copolymer, homopolymer PSBMA was synthesized using the same conditions.
1. The mixture was stirred in an inert nitrogen atmosphere and heated at 50 °C in an oil bath. After 0.5 h, VA-044 dissolved in 1 mol L−1 NaCl solution (10 mL) was injected using a syringe. The reaction proceeded for 7 h. After cooling the final reaction mixture, the copolymer was precipitated by dropwise addition into excess ethanol, and subsequent dissolution–precipitation for three times. The final precipitate obtained was put in a Petri dish and then was stored in a refrigerator at −30 °C for about 24 h. After completely frozen, the sample was then transferred it into a vacuum freeze drier (FD-1-50, Beijing Boyikang Laboratory Instruments Co., Ltd) at −51 °C, 15 Pa for 12 h. At last, the dry product was obtained. In contrast with the copolymer, homopolymer PSBMA was synthesized using the same conditions.
Molecular weights (Mw) and distributions of P(SBMA–GMA) and PSBMA were characterized via gel-permeation chromatography (GPC) measurements at 25 °C. The eluent was 0.1 mol L−1 NaNO3, with a flow rate of 0.8 mL min−1. The results are shown in Table 1. The mass percentage of the GMA monomer in the copolymer was approximately 9%.
| Sample | Mw (g mol−1) | Polydispersity index |
|---|---|---|
| PSBMA | 1.1416 × 104 | 1.32 |
| P(SBMA–GMA) | 13.6179 × 104 | 1.12 |
2.3 Fluorescence measurement
Before adding the polymer solution, a sufficient amount of pyrene solution, which was first dissolved in methanol, was evaporated in 10 mL volumetric flasks under a nitrogen stream. The resulting pyrene concentration was 5 × 10−7 mol L−1 after adding the polymer solution.32 The mixed solutions were allowed to equilibrate for 24 h with shaking prior to fluorescence runs. Then the sample was scanned for emission spectra (λex = 335 nm), which was obtained by averaging three scans, and corrected for scatter using equivalent blank polymer sample. Steady-state fluorescence measurements were performed on a Pekin-Elmer LS-55 FL spectrofluorometer at 25 °C with an excitation wavelength of 334 nm.2.4 TEM measurement
The morphologies of association micelles in the aqueous solution were observed using a JEOLIEM-1200 TEM instrument with an accelerating voltage of 60 kV. The samples were placed on copper grids coated with films at different concentrations. After the solvent completely evaporated, the measurement was carried out.2.5 Dynamic light scattering (DLS) and static light scattering (SLS) measurement
All the solutions used in DLS and SLS measurements were prepared by dissolving different amounts of P(SBMA–GMA) copolymer in 1 mol L−1 NaCl aqueous solution. After stirring for three days, all solutions were filtered through Millipore 0.45 μm membranes and were then transferred into special bottles for DLS or SLS measurement. Subsequently, the experiments were carried out using a Brook-haven instrument (USA).2.6 Zeta-potential measurement
The zeta-potential ζ, which is a parameter used to study electrostatic interactions, was measured using a Zetasizer Nano Series (Malvern) for a series of polymer solutions with different NaCl concentrations.2.7 Rheological test
All rheological measurements were performed using an AR-G2 rheometer (TA Instruments, USA), a stainless steel cone (40 mm diameter, 2°) and a plate. For the rheological test, the solutions were prepared by stirring for at least 24 h to obtain apparent homogeneity. The strain was set to 2% for frequency sweep tests in the linear viscoelastic region of the copolymer and homopolymer aqueous solutions.3 Results and discussion
3.1 Fluorescence characteristics
Complementary fluorescence techniques have been widely applied to explore the aggregate properties of associative polymers.38,39 Usually, five emission peaks in the fluorescence spectra are at the 373, 379, 384, 394, and 480 nm. The intensity ratio (I1/I3) of the first peak (I1) to the third peak (I3) is sensitive to hydrophobic environment.32,40 The highest value of I1/I3 is approximately 1.87 in water, and this value decreases in a hydrophobic environment.40 The decrease in I1/I3 relative to pure water (without polymer) reflects the slight hydrophobicity of the polymeric backbone despite the presence of hydrophilic ionic sites.39 Fig. 1 presents the variation in I1/I3 as a function of P(SBMA–GMA) concentration (cP(SBMA–GMA)) in water. The curve shows the characteristic sigmoidal shape of three regions. When cP(SBMA–GMA) is very low, pyrene senses the hydrophilic surrounding, I1/I3 approaches the value recorded in the pure solvent. At a specific cP(SBMA–GMA), I1/I3 starts to decrease, and the decline is not as steep as that observed in the case of surfactant solutions because the range of the two-order magnitudes also change with concentration. This behavior reflects the pyrene partition between the aqueous phase and the hydrophobic domains, which indicates that hydrophobic interactions exist in the P(SBMA–GMA) aqueous solution. These interactions are enhanced with increasing cP(SBMA–GMA). | ||
| Fig. 1 Fluorescence intensity ratio I1/I3 of pyrene probe as a function of cP(SBMA–GMA) at an excitation wavelength of 334 nm. | ||
3.2 P(SBMA–GMA) morphology in aqueous solution
The morphology of P(SBMA–GMA) in aqueous solution with different cP(SBMA–GMA) values were observed via TEM (Fig. 2). From Fig. 2, the P(SBMA–GMA) macromolecular chains form micelles at both extremely low (0.001 g L−1) and high concentrations (1 g L−1), in which the average micelle diameters increase with increasing cP(SBMA–GMA). The aggregation of hydrophobic blocks (GMA) is believed to cause the formation of these micelles with the hydrophilic blocks (PSBMA) as the shell.41 The micelle sizes in 1 g L−1 (Fig. 2(c)) are only a little larger than that of 0.01 g L−1 (Fig. 2(b)). However, the micelles with larger sizes are dominant in the case of 1 g L−1.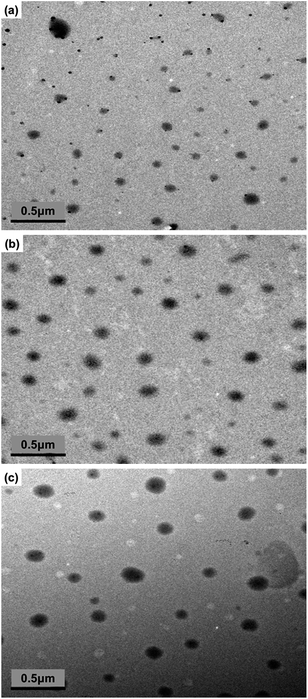 | ||
| Fig. 2 Morphology of P(SBMA–GMA) in an aqueous solution at different concentrations: (a) 0.001; (b) 0.01; (c) 1 g L−1. | ||
For most polyelectrolytes with a hydrophobic backbone, the electrostatic repulsion, whether weak or strong, favors chain stretching. Increasing the amount of salts generally results in repulsion screening and eventually provokes the collapse,42 and sometimes, the precipitation of the backbone. On the contrary, Coulombic attraction between ammonium and sulfonate groups of opposite charges along the polymer backbone hinders the dissolution of polyzwitterions in water. Coulombic attraction may be inter- or intramolecular, which results in an attraction between ammonium and sulfonate of the same or different monomers or polymer chains.43 Salts that are added in the solution may screen this kind of attraction. However, polyzwitterions, which bear both charges on the same monomeric unit and are thus virtually neutral, should not be highly sensitive to salt concentration compared with negatively and positively charged polyelectrolytes. Nevertheless, the solubility of P(SBMA–GMA) increases in NaCl aqueous solution. Even so, P(SBMA–GMA) still forms micelles in NaCl aqueous solution because of the introduction of hydrophobic blocks.44 In addition, the TEM image of P(SBMA–GMA) in NaCl aqueous solution can not be obtained because of the formation of NaCl crystals when water is evaporated. Fig. 3 presents the P(SBMA–GMA) micelle diameters in 1 mol L−1 NaCl aqueous solutions obtained via DLS. At cP(SBMA–GMA) = 0.01 g L−1, the micelle diameters are from 20 nm to 700 nm. At cP(SBMA–GMA) = 1 g L−1, the sizes vary from 20 nm to 400 nm, which have a relatively narrower diameter distribution than that in the case of cP(SBMA–GMA) = 0.01 g L−1. It is noted that both of the samples exhibit bimodal distributions. In the dissolution process, the hydrophobic sections are associated together, and the hydrophilic sections should be outside the micelles.42 However, as both of the samples are in dilute solution regime (which can be proved by Fig. 6), micelles with different sizes can't overlap with each other. Therefore, the smaller micelles are those with a relatively small amount of hydrophobic association, which may be primarily the intramolecular associations, and the bigger ones are those with a large amount of hydrophobic associations, which may contain both the intra- and intermolecular associations.45
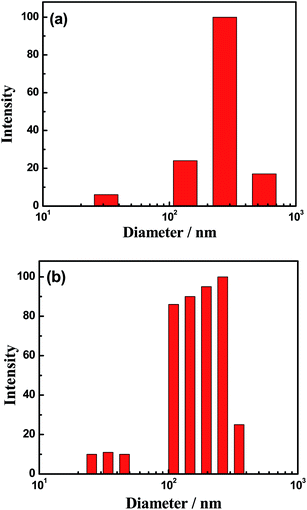 | ||
| Fig. 3 Distribution of P(SBMA–GMA) micelles in an NaCl aqueous solution (NaCl concentration is 50 g L−1) at different copolymer concentrations at 25 °C: (a) 0.01 and (b) 0.1 g L−1. | ||
3.3 Zeta-potential measurement
Fig. 4 shows the PSBMA and P(SBMA–GMA) zeta-potential (ζ) data in various NaCl aqueous solutions (cNaCl). PSBMA has an apparent negative zeta-potential (ζPSBMA) of −25.16 mV in water. P(SBMA–GMA) also has an apparent negative zeta-potential (ζP(SBMA–GMA)) of −23.6 mV in water, which is slightly higher than that of PSBMA because of the introduction of GMA. This result is in fact a general feature since all salt-free polysulfobetaine had negative zeta-potentials.22 From Fig. 5, ζPSBMA and ζP(SBMA–GMA) increase with increasing cNaCl. The result implies that Na+ interacts very strongly with –SO3− than Cl− does with (CH3)N+.43,46 Based from the research conducted by Mary, the apparent negative zeta-potential is probably consistent with the partial hydrolysis of acrylic acid functional groups.22 The preferential complexation of SBMA segment with Na+ results in an increased zeta-potential because the positive charge of Na+ compensates for the net anionic charge of partially hydrolyzed SBMA units. In other words, the sulfonated sites can have a stronger interaction with the added cation than the interaction between quaternized ammonium sites and the anions. In addition, the net negative charges significantly promote the P(SBMA–GMA) and PSBMA solubilities because of the repulsive contribution among uncompensated negative charges.12 Compared with pure PSBMA, the introduction of GMA results in an insignificant change in response to cNaCl. | ||
| Fig. 4 Zeta-potential as a function of the NaCl salt concentration measured on PSBMA and P(SBMA–GMA) solutions at a constant polymer concentration of 20 g L−1. | ||
 | ||
| Fig. 5 Shear rate dependence of the apparent viscosity of different P(SBMA–GMA) aqueous solution concentrations at 25 °C. | ||
3.4 Rheological behaviors
Viscoelastic behaviors are strongly influenced by hydrophobic association.31 In the present paper, the rheological behavior of PSBMA and P(SBMA–GMA) aqueous solution with different concentrations were investigated. As mentioned above, the P(SBMA–GMA) aqueous solution is actually a heterogeneous system due to hydrophobic block aggregation. Fig. 5 shows the apparent viscosity (ηa) versus shear rate (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) for P(SBMA–GMA) aqueous solution with various cP(SBMA–GMA). For P(SBMA–GMA) solutions with a high concentration, obvious shear-thinning was observed with increasing
) for P(SBMA–GMA) aqueous solution with various cP(SBMA–GMA). For P(SBMA–GMA) solutions with a high concentration, obvious shear-thinning was observed with increasing ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . No shear-thickening behavior was observed presumably because of low Mw and low hydrophobic block content (∼9%), which is different from other hydrophobically associating polyampholytes systems.47,48
. No shear-thickening behavior was observed presumably because of low Mw and low hydrophobic block content (∼9%), which is different from other hydrophobically associating polyampholytes systems.47,48
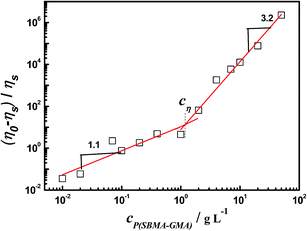
The specific viscosity (ηsp) of P(SBMA–GMA) aqueous solution with various cP(SBMA–GMA) was obtained using the equation ηsp = (η0 − ηs)/ηs, where η0 is the zero-shear viscosity and ηs is the solvent viscosity (i.e., water). In the case of high cP(SBMA–GMA), ηa at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) of 10−3 s−1 is considered as zero-shear viscosity because no η0 is observed even if
of 10−3 s−1 is considered as zero-shear viscosity because no η0 is observed even if ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) is as low as 10−3 s−1. The variations of ηsp as a function of cP(SBMA–GMA) plot in water are shown in Fig. 6. ηsp increases slightly at low cP(SBMA–GMA) and rises steeply beyond a critical concentration, cη. The crossover between the two regions is well defined, cη ≈ 1.1 g L−1. At cP(SBMA–GMA) < cη, ηsp ∝ c1.1, while ηsp ∝ c3.2 at cP(SBMA–GMA) > cη. These two regions are then considered to be dilute solution and semidilute solution respectively. Different from the polymer coils in typical neutral polymer dilute solution, there are some isolated micelles formed by intra- and intermolecular associations in dilute P(SBMA–GMA) aqueous solution as shown in Fig. 2. Some other groups found the crossover concentration between the dilute and the semidilute regime is most of the time equivalent to the critical overlap concentration (c*), meanwhile, the scales of viscosity versus concentration of the modified and unmodified polymer in dilute regime are similar.49,50 Colby's result shows that for both the polyelectrolyte and neutral polymer solutions in dilute regime, ηsp ∝ c1.0 can be found,51 which is approximately consistent with Fig. 6. At semidilute solution, most of these associative aggregate micelles can overlap with each other, and the viscosity increase sharply with concentration.52 It is hard to differentiate the semidilute unentangled regime and semidilute entangled regime in the present case. What we can infer is that the hydrophobic aggregation leads to the formation of micelles that grow with increasing concentration until at a critical concentration they percolate into a space spanning transient network.49,53
is as low as 10−3 s−1. The variations of ηsp as a function of cP(SBMA–GMA) plot in water are shown in Fig. 6. ηsp increases slightly at low cP(SBMA–GMA) and rises steeply beyond a critical concentration, cη. The crossover between the two regions is well defined, cη ≈ 1.1 g L−1. At cP(SBMA–GMA) < cη, ηsp ∝ c1.1, while ηsp ∝ c3.2 at cP(SBMA–GMA) > cη. These two regions are then considered to be dilute solution and semidilute solution respectively. Different from the polymer coils in typical neutral polymer dilute solution, there are some isolated micelles formed by intra- and intermolecular associations in dilute P(SBMA–GMA) aqueous solution as shown in Fig. 2. Some other groups found the crossover concentration between the dilute and the semidilute regime is most of the time equivalent to the critical overlap concentration (c*), meanwhile, the scales of viscosity versus concentration of the modified and unmodified polymer in dilute regime are similar.49,50 Colby's result shows that for both the polyelectrolyte and neutral polymer solutions in dilute regime, ηsp ∝ c1.0 can be found,51 which is approximately consistent with Fig. 6. At semidilute solution, most of these associative aggregate micelles can overlap with each other, and the viscosity increase sharply with concentration.52 It is hard to differentiate the semidilute unentangled regime and semidilute entangled regime in the present case. What we can infer is that the hydrophobic aggregation leads to the formation of micelles that grow with increasing concentration until at a critical concentration they percolate into a space spanning transient network.49,53
In addition, it is noted that shear thinning is observed even at cP(SBMA–GMA) < 1.1 g L−1, which is quite different from typical dilute polymer solutions. From Fig. 2 we know that the micelles with different sizes have already formed in dilute regime. Micelles with large sizes would suffer shearing and lead to the viscosity decrease with increasing shear rate.
Fig. 7 shows the dynamic rheological behavior of PSBMA and P(SBMA–GMA) aqueous solution at 100 g L−1. From Fig. 7(a), the storage (G′) and loss moduli (G′′) increase with increasing test frequency (ω) for both solutions, which is consistent with those obtained from common polymer solutions. The PSBMA aqueous solution at 100 g L−1 concentration has a higher G′′ than G′ over the test ω range. However, G′ is dominant over G′′ for the P(SBMA–GMA) aqueous solution in the test ω process despite having the same concentration as PSBMA, and the solution behaves like a weak gel. The hydrophobic association of GMA blocks is believed to enhance the interaction between the micelles and then favor the weak-gel formation.48 Another technique to confirm the formation of weak gels is to focus on the phase angle (δ) and by applying the formula tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′. When δ ≤ 45°, the samples behave like a gel. By contrast, the samples maintain a solution state at δ > 45°.48 As shown in Fig. 7(b), δ of P(SBMA–GMA) is about 10° and remains constant over the test ω scope; however, δ of PSBMA has a high value and only exhibits a value lower than 45° at high ω.
δ = G′′/G′. When δ ≤ 45°, the samples behave like a gel. By contrast, the samples maintain a solution state at δ > 45°.48 As shown in Fig. 7(b), δ of P(SBMA–GMA) is about 10° and remains constant over the test ω scope; however, δ of PSBMA has a high value and only exhibits a value lower than 45° at high ω.
 | ||
| Fig. 7 (a) Frequency dependence of the storage (G′) and loss moduli (G′′) of the P(SBMA–GMA) and PSBMA solutions at 100 g L−1. (b) Phase angle δ as a function of frequency for the same data. | ||
Another interesting feature that was investigated involves the reversibility of the rheological measurement during increasing and decreasing shear process. For PSBMA, the samples exhibit a classical rheological behavior, i.e., the viscosity is maintained constant at relatively low ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ,27 and subsequent shear thinning at
,27 and subsequent shear thinning at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) > 102 s−1 occurs. The curves obtained during increasing and decreasing ramps are fully superimposed with each other (Fig. 8). The P(SBMA–GMA) copolymer solutions, which were prepared via micellar copolymerization, behave differently. Shear thinning occurs pronouncedly even at extremely low
> 102 s−1 occurs. The curves obtained during increasing and decreasing ramps are fully superimposed with each other (Fig. 8). The P(SBMA–GMA) copolymer solutions, which were prepared via micellar copolymerization, behave differently. Shear thinning occurs pronouncedly even at extremely low ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . It is noted that the P(SBMA–GMA) aqueous solution curves obtained during increasing and decreasing ramps are also fully superimposed with each other, which is similar to that of PSBMA aqueous solution. Such viscosity decrease and recovery phenomena are usually related to the disentangling and re-entangling of polymer chains, however, it may be different for P(SBMA–GMA) aqueous solution. The recovery in viscosity for the P(SBMA–GMA) aqueous solution during decreasing
. It is noted that the P(SBMA–GMA) aqueous solution curves obtained during increasing and decreasing ramps are also fully superimposed with each other, which is similar to that of PSBMA aqueous solution. Such viscosity decrease and recovery phenomena are usually related to the disentangling and re-entangling of polymer chains, however, it may be different for P(SBMA–GMA) aqueous solution. The recovery in viscosity for the P(SBMA–GMA) aqueous solution during decreasing ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ramp test is perhaps caused by P(SBMA–GMA) large associative micelles re-entangling. And the disentangling among micelles, rather than the destruction of little hydrophobic aggregates, cause shear thinning at low
ramp test is perhaps caused by P(SBMA–GMA) large associative micelles re-entangling. And the disentangling among micelles, rather than the destruction of little hydrophobic aggregates, cause shear thinning at low ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) for the P(SBMA–GMA) solution during increasing
for the P(SBMA–GMA) solution during increasing ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ramp test. Based on the SLS results, the micelle gyration radius, Rg, of a micelle formed by a P(SBMA–GMA) chain is 97 nm. The characteristic relaxation time, τ, then can be estimated using the equation τ ≅ ηsRg3/kT, where k is the Boltzmann constant and T is the absolute temperature. For a micelle formed by a P(SBMA–GMA), τ is about 2.4 × 10−4 s. The critical shear rate for such micelle is 4.2 × 103 s−1. The maximum shear rate measured by a rheometer is only 1 × 103 s−1. Therefore, shear could only result in the transformation of large micelles to increasingly smaller ones through disentangling. However, this process could not destroy the little hydrophobic aggregates, which requires a relatively longer recovery time.54
ramp test. Based on the SLS results, the micelle gyration radius, Rg, of a micelle formed by a P(SBMA–GMA) chain is 97 nm. The characteristic relaxation time, τ, then can be estimated using the equation τ ≅ ηsRg3/kT, where k is the Boltzmann constant and T is the absolute temperature. For a micelle formed by a P(SBMA–GMA), τ is about 2.4 × 10−4 s. The critical shear rate for such micelle is 4.2 × 103 s−1. The maximum shear rate measured by a rheometer is only 1 × 103 s−1. Therefore, shear could only result in the transformation of large micelles to increasingly smaller ones through disentangling. However, this process could not destroy the little hydrophobic aggregates, which requires a relatively longer recovery time.54
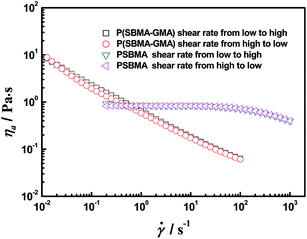 | ||
| Fig. 8 Apparent viscosity as a function of shear rate for copolymer and homopolymer solutions at 25 °C at a concentration of 50 g L−1. | ||
The effect of temperature on the rheological behavior of hydrophobic association polymers is significant for applications. Fig. 9 shows the temperature dependence of viscosity for copolymer and homopolymer aqueous solutions. For PSBMA, the viscosity decreases with gradual increases in temperature and increase with decreasing temperature. Note that the data from the procedure are in accordance with those obtained using a conventional neutral polymer solution temperature ramp procedure.55 The Arrhenius equation can be used to depict temperature dependence of the PSBMA aqueous solution viscosity, as shown in Fig. 9(a). The Arrhenius equation is stated as,
η = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eΔEη/RT eΔEη/RT
| (1) |
Many research have focused on the salt concentration dependence of viscosity for polyampholyte solutions.59,60 Several researchers have pointed out that salt ions could affect the chain conformations to screen the repulsion between excess charges and the attraction between opposite charges.12,26 Schulz found that polysulfobetaine solutions display an antipolyelectrolyte behavior, i.e., viscosities that increase with increasing salt concentrations.61 In the current study, the relationship between salt concentration and the viscosity of hydrophobically modified PSBMA solution is shown in Fig. 10. The viscosity initially decreases and then increases when more salt was added. A critical point in the curves is observed. To explain this phenomenon, we recourse to the theory proposed by Mary.22 The Debye length lD is defined as,62
| lD = 0.3/[NaCl]1/2 | (2) |
| lD± = (4a3N1/2)1/3 | (3) |
| c± ≈ (3/2πa3)N1−3β | (4) |
 | ||
| Fig. 10 NaCl salt concentration dependence of viscosity for P(SBMA–GMA) at a shear rate of 0.1 s−1 at 20 g L−1 and 25 °C. | ||
| cNaCl (g L−1) | 0 | 0.1 | 1 | 10 | 100 |
| lD (nm) | — | 7.25 | 2.29 | 0.72 | 0.23 |
According to Mary, β is the power exponent associated with the solvent quality for the polymer chain. In the current study, β should be equal to 1/2. For P(SBMA–GMA) copolymers, Mw = 1.4 × 105; therefore, N is approximately equal to 400. We can then estimate c± is to be approximately 0.08 mol L−1. This value is almost consistent with the experimental data at 1 g L−1, which is approximately 0.02 mol L−1. lD± is estimated to be about 2.98 nm.
After acquiring these important parameters, we tried to explain the phenomenon in Fig. 10. From Fig. 4, the polymer solution contains uncompensated charges. In salt-free solutions, the chains are stretched because of the repulsion between the negative charges.12 When a small amount of salt is added, lD decreases but is still larger than lD± (2.98 nm). The salt can screen the repulsion between uncompensated charges with decreasing polymer chain size. At cNaCl of 1 g L−1, lD is about 2.29 nm; thus, lD is approximately equal to lD± where in the chain size has the lowest value. When more salt is added, lD is smaller than lD±; therefore, the attraction between opposite charges in polyzwitterionic coils can be screened by the added salt. As a result, the chain size increases and leads to an apparent increase in viscosity. The above result is also consistent with the theoretical model suggested by Dobrynin and Rubinstein. They discussed various regimes for polyampholyte chains with charge asymmetry in salt solutions. In a no-salt regime, the chains are stretched by excess charges. In a low-salt regime, the chains exhibit polyelectrolyte behaviors. In a high-salt regime, the chains show properties similar to those of polyampholyte.12,26
4 Conclusions
Hydrophobically modified PSBMA formed micelles even at extremely low concentrations because of hydrophobic block associations. Such hydrophobic interactions are enhanced with increasing polymer concentration. Both PSBMA with and without hydrophobic modification exhibit negative zeta-potential which increases with increasing polymer concentration. The viscosity of P(SBMA–GMA) aqueous solution can be divided into two regions, in which the concentration dependence is ηsp ∝ c1.1 and ηsp ∝ c3.2 for dilute and semidilute solutions, respectively. Quite different from typical dilute polymer solutions, P(SBMA–GMA) solution exhibits shear thinning even at cP(SBMA–GMA) < cη. Under the same high concentration, P(SBMA–GMA) solution behaves like a weak gel (G′ > G′′), while viscosity is dominant in PSBMA solution (G′ < G′′). After shearing, the solution viscosity of P(SBMA–GMA) could be immediately recovered as well as that of PSBMA. The rheological behavior of P(SBMA–GMA) solution shows an unusual relationship with temperature and G′ is much more sensitive to temperature than G′′ because of the existence of hydrophobic association and epoxy groups. Moreover, the viscosity of P(SBMA–GMA) solution is sensitive to the added salt concentration, i.e., the viscosity initially decreases and subsequently increases upon the addition of salt. The critical salt concentration is considered to be related to the Debye length lD of salt solution and to the average microscopic distance between ammonium and sulfonate charges confined inside a polyzwitterionic coil. These excellent properties provide many applications in various regions, such as repellant oil agent, cosmetic, water treatment agent, etc.Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant 20674072, 51173164).References
- K. Ishihara, H. Nomura, T. Mihara, K. Kurita, Y. Iwasaki and N. Nakabayashi, J. Biomed. Mater. Res., 1998, 39, 323–330 CrossRef CAS.
- Y. Iwasaki, S. Sawada, K. Ishihara, G. Khang and H. B. Lee, Biomaterials, 2002, 23, 3897–3903 CrossRef CAS.
- T. Nakaya and Y. J. Li, Prog. Polym. Sci., 1999, 24, 143–181 CrossRef CAS.
- G. Cheng, G. Z. Li, H. Xue, S. F. Chen, J. D. Bryers and S. Y. Jiang, Biomaterials, 2009, 30, 5234–5240 CrossRef CAS PubMed.
- Z. Zhang, J. A. Finlay, L. F. Wang, Y. Gao, J. A. Callow, M. E. Callow and S. Y. Jiang, Langmuir, 2009, 25, 13516–13521 CrossRef CAS PubMed.
- L. Carr, G. Cheng, H. Xue and S. Y. Jiang, Langmuir, 2010, 26, 14793–14798 CrossRef CAS PubMed.
- J. C. Hower, Y. He, M. T. Bernards and S. Y. Jiang, J. Chem. Phys., 2006, 125, 214704–214711 CrossRef PubMed.
- B. D. Ratner and S. J. Bryant, Annu. Rev. Biomed. Eng., 2004, 6, 41–75 CrossRef CAS PubMed.
- R. Langer, Science, 2001, 293, 58–59 CrossRef CAS PubMed.
- K. L. Prime and G. M. Whitesides, Science, 1991, 252, 1164–1167 CrossRef CAS.
- A. Ohlemacher, F. Candau, J. P. Munch and S. J. Candau, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 2747–2757 CrossRef CAS.
- A. V. Dobrynin and M. Rubinstein, J. Phys. II, 1995, 5, 677–695 CrossRef CAS.
- P. G. Higgs and J. F. Joanny, J. Chem. Phys., 1991, 94, 1543–1554 CrossRef CAS PubMed.
- A. Diehl, M. C. Barbosa and Y. Levin, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1996, 54, 6516–6525 CrossRef CAS.
- A. V. Dobrynin and M. Rubinstein, Prog. Polym. Sci., 2005, 30, 1049–1118 CrossRef CAS PubMed.
- A. Parker and W. Fieber, Soft Matter, 2013, 9, 1203–1213 RSC.
- M. Skouri, J. P. Munch, S. J. Candau, S. Neyret and F. Candau, Macromolecules, 1994, 27, 69–76 CrossRef CAS.
- S. V. Singh, P. K. Singh, A. V. Singh, J. S. Sohal, V. K. Gupta and V. S. Vihan, Vaccine, 2007, 25, 7102–7110 CrossRef CAS PubMed.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4189 CrossRef CAS PubMed.
- C. L. McCormick and C. B. Johnson, Polymer, 1990, 31, 1100–1107 CrossRef CAS.
- C. L. McCormick and L. C. Salazar, Polymer, 1992, 33, 4384–4387 CrossRef CAS.
- P. Mary, D. D. Bendejacq, M. P. Labeau and P. Dupuis, J. Phys. Chem. B, 2007, 111, 7767–7777 CrossRef CAS PubMed.
- G. Z. Zheng, G. Meshitsuka and A. Ishizu, J. Polym. Sci., Part B: Polym. Phys., 1995, 33, 867–877 CrossRef CAS PubMed.
- C. L. McCormick and L. C. Salazar, Macromolecules, 1992, 25, 1896–1900 CrossRef CAS.
- R. G. Ezell, I. Gorman, B. Lokitz, N. Treat, S. D. McConaughy and C. L. McCormick, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4479–4493 CrossRef CAS PubMed.
- A. V. Dobrynin, R. H. Colby and M. Rubinstein, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3513–3538 CrossRef CAS PubMed.
- A. A. Abdala, W. J. Wu, K. R. Olesen, R. D. Jenkins and S. A. Khan, J. Rheol., 2004, 48, 979–994 CrossRef CAS.
- M. A. Winnik and A. Yekta, Curr. Opin. Colloid Interface Sci., 1997, 2, 424–436 CrossRef CAS.
- R. D. Hester and D. R. Squire, J. Coat. Technol., 1997, 69, 109–114 CrossRef CAS.
- K. C. Taylor and H. A. Nasr-El-Din, J. Pet. Sci. Eng., 1998, 19, 265–280 CrossRef CAS.
- E. Volpert, J. Selb and F. Candau, Polymer, 1998, 39, 1025–1033 CrossRef CAS.
- A. Hill, F. Candau and J. Selb, Macromolecules, 1993, 26, 4521–4532 CrossRef CAS.
- S. Choudhary and S. R. Bhatia, Carbohydr. Polym., 2012, 87, 524–530 CrossRef CAS PubMed.
- O. Borisova, L. Billon, M. Zaremski, B. Grassl, Z. Bakaeva, A. Lapp, P. Stepanek and O. Borisov, Soft Matter, 2011, 7, 10824–10833 RSC.
- E. K. Penott-Chang, L. Gouveia, I. J. Fernandez, A. J. Muller, A. Diaz-Barrios and A. Eduardo Saez, Colloids Surf., A, 2007, 295, 99–106 CrossRef CAS PubMed.
- H. Chen, Z. M. Wang, Z. B. Ye and L. J. Han, J. Appl. Polym. Sci., 2014, 131(1), 39707 CrossRef PubMed.
- Y. J. Che, Y. B. Tan, J. Cao and G. Y. Xu, J. Macromol. Sci., Part B: Phys., 2010, 49, 695–710 CrossRef CAS.
- L. E. Bromberg and D. P. Barr, Macromolecules, 1999, 32, 3649–3657 CrossRef CAS.
- M. Wilhelm, C. L. Zhao, Y. C. Wang, R. L. Xu, M. A. Winnik, J. L. Mura, G. Riess and M. D. Croucher, Macromolecules, 1991, 24, 1033–1040 CrossRef CAS.
- L. E. Bromberg and D. P. Barr, Biomacromolecules, 2000, 1, 325–334 CrossRef CAS.
- Z. Iatridi and C. Tsitsilianis, Soft Matter, 2013, 9, 185–193 RSC.
- Y. J. Che, Y. B. Tan, J. Cao and G. Y. Xu, J. Macromol. Sci., Part B: Phys., 2010, 49, 695–710 CrossRef CAS.
- P. N. Nesterenko and P. R. Haddad, Anal. Sci., 2000, 16, 565–574 CrossRef CAS.
- A. S. Kimerling, W. E. Rochefort and S. R. Bhatia, Ind. Eng. Chem. Res., 2006, 45, 6885–6889 CrossRef CAS.
- A. Niu, D. J. Liaw, H. C. Sang and C. Wu, Macromolecules, 2000, 33, 3492–3494 CrossRef CAS.
- W. F. Lee and C. C. Tsai, Polymer, 1995, 36, 357–364 CrossRef CAS.
- B. Nystrom, A. L. Kjoniksen, B. Ni, K. Z. Zhu and K. D. Knudsen, Soft Matter, 2009, 5, 1328–1339 RSC.
- J. P. Celli, B. S. Turner, N. H. Afdhal, R. H. Ewoldt, G. H. McKinley, R. Bansil and S. Erramilli, Biomacromolecules, 2007, 8, 1580–1586 CrossRef CAS PubMed.
- C. Chassenieux, T. Nicolai and L. Benyahia, Curr. Opin. Colloid Interface Sci., 2011, 16, 18–26 CrossRef CAS PubMed.
- E. J. Regalado, J. Selb and F. Candau, Macromolecules, 1999, 32, 8580–8588 CrossRef CAS.
- R. H. Colby, Rheol. Acta, 2010, 49, 425–442 CrossRef CAS PubMed.
- P. Kujawa, A. Audibert-Hayet, J. Selb and F. Candau, Macromolecules, 2006, 39, 384–392 CrossRef CAS.
- M. Rubinstein and A. N. Semenov, Macromolecules, 2001, 34, 1058–1068 CrossRef CAS.
- L. Jin, Y. G. Shangguan, T. Ye, H. Yang, Q. F. An and Q. Zheng, Soft Matter, 2013, 9, 1835–1843 RSC.
- Q. Wu, M. Du, T. Ye, Y. G. Shangguan, J. P. Zhou and Q. Zheng, Colloid Polym. Sci., 2009, 287, 911–918 CAS.
- C. L. McCormick, T. Nonaka and C. B. Johnson, Polymer, 1988, 29, 731–739 CrossRef CAS.
- P. Setny, R. Baron and J. A. McCammon, J. Chem. Theory Comput., 2010, 6, 2866–2871 CrossRef CAS PubMed.
- Y. X. Zhang, A. H. Da, G. B. Butler and T. E. Hogenesch, J. Polym. Sci., Part A: Polym. Chem., 1992, 30, 1383–1391 CrossRef CAS PubMed.
- R. G. Ezell and C. L. McCormick, J. Appl. Polym. Sci., 2007, 104, 2812–2821 CrossRef CAS PubMed.
- Y. Umar, B. F. Abu-Sharkh and S. A. Ali, Polymer, 2005, 46, 10709–10717 CrossRef CAS PubMed.
- D. N. Schulz, D. G. Peiffer, P. K. Agarwal, J. Larabee, J. J. Kaladas, L. Soni, B. Handwerker and R. T. Garner, Polymer, 1986, 27, 1734–1742 CrossRef CAS.
- D. Leckband and J. Israelachvili, Q. Rev. Biophys., 2001, 34, 105–267 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |