Identification of disinfection by-product precursors from the discharge of a coking wastewater treatment plant†
Abstract
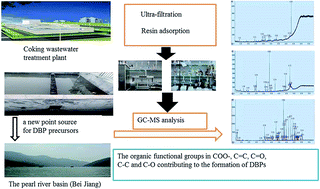
Coking wastewater discharge can lead to pollution, due to water reuse or release to surface water after the disinfection process. In this work, the dissolved organic matter (DOM) in the effluent was isolated into 42 classes using molecular weight distribution and resin adsorbents. The trihalomethane and haloacetonitrile formation potential (THMFP and HANFP) from each fraction were measured and correlated with the UV-vis absorption and fluorescence excitation-emission matrix (EEM), and the compounds in the classes as precursors were determined by solid phase extraction, silica chromatography and GC/MS. The results show that the highest SUVA in the >100 kDa faction was 12.1 L mg−1 cm−1. The lower MW fractions (5–10 kDa, 3–5 kDa, 1–3 kDa and <1 kDa) had similar SUVA of about 4.1 L mg−1 cm−1. The HiA (Hydrophilic acids) fraction was found to be the most abundant, constituting about 45% of DOC. The THMFP and HANFP show that the DOM fraction with low MW and HiA was the dominant fraction and contributed more precursors. The EEM spectra indicated there were notable amounts of soluble microbial products and aromatic proteins in the >100 kDa fraction. Based on the results of the GC/MS analysis, nitriles, amines, nitrogenous heterocyclics, hydrocarbons, polycyclic aromatic hydrocarbons, esters, phenols, alcohol, ketones, and organic acids determined were as precursors in the <1 kDa fraction. Both precursors have functional groups with high chlorine reactivity, such as carboxylate salt COO–, aromatic structures C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, aldehydes and ketones groups C
C, aldehydes and ketones groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, carbohydrates C–C and O-alky group C–O contributing greatly to the formation of disinfection by-production precursors.
O, carbohydrates C–C and O-alky group C–O contributing greatly to the formation of disinfection by-production precursors.

 Please wait while we load your content...
Please wait while we load your content...