Extraction of actinides by tertiary amines in room temperature ionic liquids: evidence for anion exchange as a major process at high acidity and impact of acid nature†
Abstract
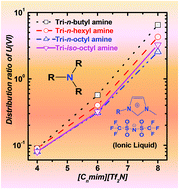
Extraction of U(VI) and Pu(IV) using several tri-alkylamines such as tri-n-butylamine (TBA), tri-n-hexylamine (THA), tri-n-octylamine (TOA), and tri-iso-octylamine (TiOA) in room temperature ionic liquids, [Cnmim][Tf2N] (where n = 4, 6 or 8), was investigated from nitric acid as well as hydrochloric acid medium. In the absence of the amines, the extraction results indicated an increase in the extraction of both U(VI) and Pu(IV) as a function of the acid concentration which was attributed to the extraction of probable anionic species such as UO2X3−, UO2X42−, PuX5− and PuX62−(where X = Cl− or NO3−) according to an anion-exchange mechanism involving Tf2N− ions. The presence of amines in the ionic liquid enhances the extraction of the metal ions with increased HCl concentration, especially in the case of UO22+, but the amines appear to be almost inefficient in HNO3 medium. This is ascribed to the protonation/association of amines via solubilization of H+ and NO3− ions in the ionic liquid phase in the case of nitric medium, while hydrochloric acid does not solubilize in ionic liquid, and thus the amine remains efficient. Modeling of the extraction data in HCl medium for U(VI) and Pu(IV) in the presence of amines has been performed and confirmed the anion exchange mechanism.

 Please wait while we load your content...
Please wait while we load your content...