A low-energy milling approach to reduce particle size maintains the luminescence of strontium aluminates
R. E. Rojas-Hernandez*,
F. Rubio-Marcos,
E. Enríquez,
M. A. De La Rubia and
J. F. Fernandez
Electroceramic Department, Instituto de Cerámica y Vidrio, CSIC, Kelsen 5, 28049, Madrid, Spain. E-mail: rociorojas@icv.csic.es; Fax: +34 91 735 58 43; Tel: +34 91 735 58 40 ext. 1074
First published on 6th May 2015
Abstract
Decreasing the particle size, improving the distribution of the particle size, avoiding a high agglomeration state and maintaining the photoluminescence response of SrAl2O4 doped with Eu2+ and Dy3+ powders is still a challenge in the processing of this phosphorescent material. Here, we explore different processes to achieve this objective. The standard route is a wet milling process, however the presence of a liquid medium promotes the hydrolysis of the material, and therefore its results are deleterious for its functional properties. These problems may be avoided if the milling is carried out by means of a dry process. For this reason, the powders are milled following two different procedures: high and low energetic dry milling processes. A correlation between the reduction of particle size and intensity of the photoluminescent emission has been evaluated. In this context, this study develops different processing routes to reduce the particle size on phosphor powders, seeking an agreement between the optical properties and the size of the powders, that depend on the final requirements.
1. Introduction
Phosphorescent inorganic materials that emit light in the visible range have been actively studied in the last decade due their applications in safety signage (signposts in roads, signs in or outside of buildings), dials, displays and night-vision surveillance.1,2 Photoluminescence is light emission as a result of absorption of photons; the decay of the light emission is no longer than a few milliseconds after the end of the excitation in fluorescence materials. On the contrary, phosphorescent materials emits light from minutes to several hours. Up to now, different types of inorganic matrices including sulfides, aluminates, silicates, titanates, oxysulfides, nitrides, pyrophosphates, etc., doped with rare earth ions have mainly been synthesized as long-phosphorescence materials. However, the strontium aluminate family hosts are more extensively used because of their excellent phosphorescence properties and good stability.3–6 After the discovery in 1996 of SrAl2O4:Eu2+, Dy3+ as a new persistent luminescent compound by Matsuzawa et al.,7 many researchers have developed some methods for preparation of these powders including a sol–gel method, hydrothermal synthesis, chemical precipitation, laser synthesis and solid-state reaction. However, the powders have large particle size that is unpractical for printing applications in which particles <10 μm are required or even beyond potential applications as luminescent labels for biomolecules and mechano-optical nano-devices that require particles in the nanometer range.Solid state reaction particles, for example, requires high temperatures, typically 1300–1900 °C, long processing time and as result the average size is ranged 20–100 μm in diameter.8–10 This procedure is used to synthesize commercial SrAl2O4:Eu2+, Dy3+ phosphors that are now in the market.
The synthesis of sub-micron phosphorescent particles has been widely studied during the past decade because of the promising industrial application of these materials. In order to overcome the limiting issues, chemical routes such as sol–gel and microemulsion synthesis have been employed. These techniques produce nanopowders homogeneous and dispersed but the persistent luminescence is significantly reduced in comparison with the micrometer powders. Therefore, these methods usually required a post-thermal treatment in order to improve the phosphorescent performance, nevertheless the final properties could not compete with the microscale products.11 Taking in account these disadvantages, the phosphorescent particle production in the sub-micron range is currently a critical objective of the industrial research. Ball milling techniques have been used in order to reduce the particle size, moreover these techniques need a liquid media that produces a high detrimental of photoluminescence performance. It is known that strontium aluminates have a weak water resistance and may absorb CO2, as a consequence a liquid media containing water is a drawback for this material class.12 Moreover, some approaches have obtained the aggregation of smaller particles after long milling times ≅ 48 hours,13 that it is not practical for industrial uses.
High-performance phosphors have been thought as one kind of very important and widely used materials, and the related researches always become frontier and hot in the field of high-technology advanced materials. Nonetheless, luminescent properties depend considerably on the grain size, crystalline size and morphology; attractive applications will be possible if the grain size decreases. Luminescent materials exhibit attractive properties when the grain size reaches the nanometer scale, when sizes become comparable to their Bohr excitonic radius.14 As a result, “particle size” plays an important role in the whole development of Strontium Aluminates phosphors. Generally speaking, the luminescent activity of SrAl2O4:Eu, Dy-nano phosphors is still inferior to those of most SrAl2O:Eu, Dy-microstructured, and thus the further breakthrough must be achieved for extensively replacing the micrometric-based ones in some applications. Nanometer scale implies a great increment of the surface energy that has a huge influence on the crystal field and atom structure around the Eu2+. These changes cause for example a blue shift in the excitation and emission that could be interesting for some applications and a lower initial intensity compared with the microscale analogous.15
For that, the aim of this work is to explore milling methods to reduce the size of a commercial SrAl2O4:Eu2+, Dy3+ phosphor as raw material. To carry out this top-down method, the phosphor particles are milling in an attritor mill using ethanol or by following dry milling processes. The dry milling is proposed to avoid the contact of the powder with liquid media and to minimize the moisture content. The low energy dry milling is used to disperse nanoparticles in microparticles16,17 and the high-energy milling, which is also known as the mechanical alloying, is used to synthesize compounds.18,19 However when applying this last process to previous synthesized powder, the high-energy milling in short periods mainly reduces the crystallite and particle size20 and no chemical reaction occurs at this stage, for this reason this method offers a very attractive advantage to reduce greatly the size of the materials.
The characteristics of the powder after milling are modified due to the effective parameters in the milling process, rotation speed, milling time, type and size of the container and the milling balls, milling media and atmospheric conditions. The energy transferred in the milling process is related mainly to the frequency, time, type of balls and container employed. Moreover, to avoid chemical transformation it is important to properly select the balls, the milling media and the atmosphere.21,22 To take into account these variables in SrAl2O4 system different parameters will be studied.
Herein, we will describe the effect of the milling on the properties of the SrAl2O4. We focus on the design and the preservation of the intrinsic structure in strontium aluminates materials by different routes to reduce the particle size. The main achievements are related to use a method to tailor the particle size, without damaging the potential optical properties of this material.
2. Experimental details
Reconditioning the particle size
A commercial SrAl2O4:Eu2+, Dy3+ phosphor powder (SAO) from Jinan Chenghao technology co., Ltd was milled by three different procedures. In the first method, the powders were attrition-milled by using cerium-stabilized zircona balls (1 mm) in a polyethylene attritor mill in ethanol for 3 and 8 h, being the charge ratio (mass of grinding balls: mass of powder in mill) equal to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the ethanol media filled 33% of the container. Then the milled powder was dried at 100 °C for 5 h. In the second method the powder high energetically milled with a Pulverisste 5 model Fritsch planetary mill operating at 250 rpm by using wolfram carbide (WC) equipped with cylindrical hardened steel container (250 cm3) and WC balls for 5, 10 and 25 minutes in air atmosphere; being the charge ratio (mass of grinding balls: mass of powder in mill) equal to 20
1, the ethanol media filled 33% of the container. Then the milled powder was dried at 100 °C for 5 h. In the second method the powder high energetically milled with a Pulverisste 5 model Fritsch planetary mill operating at 250 rpm by using wolfram carbide (WC) equipped with cylindrical hardened steel container (250 cm3) and WC balls for 5, 10 and 25 minutes in air atmosphere; being the charge ratio (mass of grinding balls: mass of powder in mill) equal to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In the third method, a low energy dry milling process has been carried out with a turbula-type mixer by using cerium-stabilized zirconia balls (0.5 mm) in a 60 cm3 nylon container for 5 min, 10 min, 20 min and 40 min at 50 rpm in air atmosphere; being the charge ratio (mass of grinding balls: mass of powder in mill) equal to 1
1. In the third method, a low energy dry milling process has been carried out with a turbula-type mixer by using cerium-stabilized zirconia balls (0.5 mm) in a 60 cm3 nylon container for 5 min, 10 min, 20 min and 40 min at 50 rpm in air atmosphere; being the charge ratio (mass of grinding balls: mass of powder in mill) equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Structural and microstructural characterization
In order to follow the evolution of the phase of the powders with milling-time, the powders were characterized by X-ray diffraction (XRD, D8, Bruker) using Cu Kα radiation. Rietveld refinement was performed using the general structure analysis system (GSAS) program with the EXPGUI graphical user interface23,24 in order to obtain the full width-at half-maximum (FWHM) of the diffraction peaks. For the profile fitting, the modified Tompson–Cox–Hastings pseudo-Voigt (TCH-pV)25 function was used. The crystallite size was extracted from the refined profile parameters.26 The (Θ)-independent Gaussian term (GW), and (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Θ) and (tan
Θ) and (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Θ)-dependent Lorentzian terms (Lx and LY, respectively) were refined.27 Unit cell parameters, scale-factors, background and coefficients corresponding displacement and zero-shift correction were also refined. Particle size distribution measurements of the phosphor powders were determined by laser diffraction (Mastersizer S, Malvern, U.K.). Measurements of d50 (average particle size), were made. The morphology of powders was evaluated using secondary electron images of field emission scanning electron microscopy (FE-SEM, Hitachi S-4700).
Θ)-dependent Lorentzian terms (Lx and LY, respectively) were refined.27 Unit cell parameters, scale-factors, background and coefficients corresponding displacement and zero-shift correction were also refined. Particle size distribution measurements of the phosphor powders were determined by laser diffraction (Mastersizer S, Malvern, U.K.). Measurements of d50 (average particle size), were made. The morphology of powders was evaluated using secondary electron images of field emission scanning electron microscopy (FE-SEM, Hitachi S-4700).
Luminescent characterization
Optical properties of these materials were investigated by measuring emission and excitation spectra. The photoluminescence spectra of the phosphor particles were recorded with a spectrofluorometer (Fluorolog®-3, HORIBA Jobin Yvon) at room temperature. The luminescence intensity was measured over the wavelength 425–650 nm, a Xenon arc lamp was used as an excitation source (λexc = 380 nm)3. Results
Wet-milling processing by attrition milling: a drawback for the SrAl2O4:Eu2+, Dy3+ phosphor
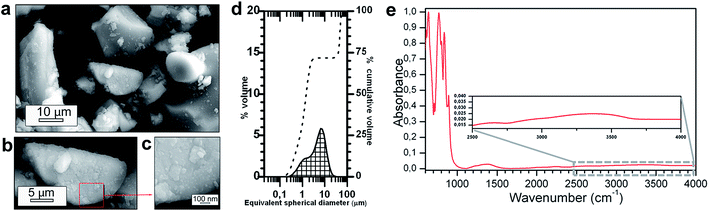
The morphological nature of the as received commercial powder used can be seen from Fig. 1a–c. The powder is formed by particles ranging from 8 to 40 μm; the particles show an irregular shape. In Fig. 1b, it can be observed that the particles are dense and have sharp fracture surfaces that resembles mirror surface as consequence of previous grinding process. A more exhaustive inspection of the morphology reveals that at the surface small grains having hundreds of nanometers are presents. The surface nanostructured seems to be form a smooth layer by a reaction after the grinding; see Fig. 1c. The average particle size is d50 ∼ 6.7 μm and its particle size distribution has a bimodal distribution (see Fig. 1d).Wet-milling approach is one of the leading process for particle size reduction widely used, employing generally water as a media. However, strontium aluminates are too sensitive to water. The FTIR spectra for the commercial powder is shown in Fig. 1e. A broad band appears at 3374 cm−1 (Fig. 1e inset) corresponding to vibrations of free and hydrogen-bonded hydroxyl groups due to the air exposure.28 This band is weak, therefore it suggest that the surface of phosphors has low water absorption from the environment. The band located at 1381 cm−1 could be assigned to strontium carbonate formed.29 The presence of these bands corroborates the SAO affinity for water by the environmental exposure. Apparently, the surface layer created is characterized by their nanostructure character, as previously mentioned, and it is a consequence of the SAO reaction with the moisture. In the 1000–600 cm−1 spectral range, there are multiples bands corresponding to the characteristic vibrations of Al–O, Sr–O and Sr–O–Al bonds. A detailed study previously done for SrAl2O4:Eu2+, Dy3+ compounds attributed the presence of peaks at 842, 785, 655 and 639 cm−1 to some stretching vibrations of the AlO4 tetrahedra and 893, 869, 803, 768, 711, 672 and 612 cm−1 to the Al–O stretching modes in SrAl2O4.30
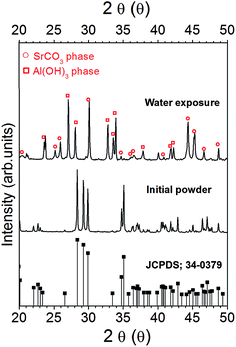
As shown in the bottom of Fig. 2, the XRD pattern of commercial SrAl2O4:Eu2+, Dy3+ phosphor powder exhibits characteristic peaks of the SrAl2O4 monoclinic phase (space group P21), whose pattern is characterized by three peaks centered in the 2θ range 28° to 30° and matched with the SrAl2O4 standard values give in JCPDS (no. 34-0379).
When the SAO material is immersed in water an hydrolysis reaction occurs, which corroborates that this phase is not stable in water. As it can see in the upper XRD in Fig. 2 after reaction of SrAl2O4 powder with water, aluminium hydrate phase (Al(OH)3) appeared together with SrCO3 phase. The phenomenology described here, could be explained by a simple decomposition mechanism based on the hydrolysis reaction of the SrAl2O4 phase that evolves towards the formation of Al(OH)3 and SrCO3. As a result, we can detail the decomposition mechanism of the SAO upon H2O exposure. The first one is associated to water incorporated to the hydrolysis reaction,12 while the second one corresponds to the absorb CO2 (ref. 31) which are present at ambient atmosphere with SrO, such as shown the eqn (1) and (2), respectively.
| SrAl2O4 + 4H2O → Sr(OH)2+ 2Al(OH)3 | (1) |
| Sr(OH)2 + CO2 → SrCO3 + H2O | (2) |
To avoid the exposure to water and as a consequence, the secondary phases appearance, we decided to use absolute ethanol as a milling medium.
To obtain particles in submicron sizes, the powders are milled by attrition process. It is interesting to note that each material has a different behaviour and it is therefore necessary to optimize the operating parameters. In our efforts to reduce the particle size, we propose two milling times, such as 3 and 8 hours. After milled for 3 h, the powders have a median particle size d50 ∼ 5.0 μm. This result indicates that no greatly reduction has been achieved. In order to attain the efficiency of the milling, increasing the milling time is here proposed. So, prolonged ball-milling (8 h) implies a significantly reaction with the media, XRD patterns in Fig. 3a reveal the damage of the monoclinic structure. The powder milled for 8 hours has a median particle size d50 ∼ 3.9 μm. As a consequence, the most probable origin of this behaviour must be related to the increase of the available surface area during the milling, that implies a higher surface exposure to moisture and higher number of active defects, which start the hydrolysis process (as we have proposed in the eqn (1) and (2)).
As it has been explained in the introduction section, the luminescent properties depend considerably on the particle size and/or its crystalline structure; attractive applications will be possible if the particle size decreases. To verify that the luminescent properties are strongly influenced by the particle size and the structure of the Sr0,97Al2O4:Eu0,02, Dy0,01 phase. Thus, we proceed to evaluate the functional properties of the milled material in wet conditions. Fig. 3b shows the photoluminescence emission spectra of commercial SrAl2O4:Eu, Dy powder and after milling process during 3 and 8 hours, upon excitation at 380 nm. The emission band centered at 510 nm are assigned to the spin-allowed transition of 4f65d1 → 4f7 (8S7/2) of Eu2+ ions,32 The results reveal a significantly detrimental photoluminescent performance. The inset in Fig. 3b reveals a emission located at 611 nm correspond to the spin-forbidden transition of 5D0 → 7F2 for the Eu3+ ions,33 such origin is attributed to the damage of the particle surface provoking the amorphization of the SrAl2O4:Eu2+, Dy3+ particles, which may produce the partial oxidation of the Eu2+ to Eu 3+ ions.
To sum up, we can conclude that this milling process is inefficient to produce sub-micron particles; the disadvantage of this route is the lack of control of the potential reactive surface that implies the deterioration of the material and their poor photoluminescence. This means that a wet-milling is not allowable for these materials because their luminescent properties are very sensitive to the chemical reaction with the milling media. In order to minimize the damage during the milling process, we propose a dry milling-based method, and the results will be presented in the next section.
Dry processing by high-energy and low-energy ball-milling
According to the above results, there are another procedure to be used for the size reduction of solid particles such as dry milling process, which shall avoid the degradation of the material. In the first part, we will evaluate the ability to reduce the particle size by high-energy dry milling (HDM), as well as the influence on the phase structure and consequently its functional properties of the SrAl2O4:Eu, Dy phosphor. In the second part, we suggest the low energetic-dry-ball-milling process (LDM) as an adequate method for further improving photoluminescence on these materials.Although structural information on the SrAl2O4:Eu2+, Dy3+ phosphors obtained by XRD is valuable, the XRD technique yields very limited microstructural information. So, the milling time effect on the morphology of the SrAl2O4:Eu2+, Dy3+ phosphors is also evaluated by FE-SEM technique. The morphology of SrAl2O4:Eu2+, Dy3+ powder after high-energetic milling during 25 min are shown in Fig. 5. As shown in Fig. 5a, the powders show small and uniform particle dimensions. These results demonstrate that a broad particle size distribution is clearly due to the agglomeration state. The primary particle size is decreased by the milling-process but the agglomeration state becomes more marked as the milling time increases. While the primary particle size appeared to have been successfully reduced by the HDM, the surface of the particles is altered, generating the defects formation at the particle surface, as shown in the Fig. 5c and d. These defects are caused by impact energy produced during the HDM, provoking the generation of highly reactive surfaces, as signaled with yellow arrows.
The main disadvantages of HDM process is the agglomeration state of the particles and the damage of the surface generated by the high energy impacts. For this reason, a low energetic-dry milling process is proposed to attempt the reduction of particle size without agglomeration state of the powders. The effectiveness of the process is evaluated in the next section.
It is important to remark that the milling energy influence on the evolution of particle reduction process. Commonly, the increase of mass of balls/mass of powder ratio and the mill frequency implies a faster amorphization of the structure.34 The mill frequency and the mass of balls/mass of powder ratio of the High-energy dry milling (HDM) is five times and twenty times higher, respectively than the Low-energy dry milling (LDM), therefore the energy transferred in HDM process accelerates the particle surface damage.
Fig. 7a–d show the micrographs obtained by FESEM of the commercial powder after LDM during 40 min. It can be seen that the average size of the particles is ∼2.8 μm, this result is in good agreement with the sizes obtained in particle size distribution. Moreover, an added advantage of this method is the dispersion of the particles during the milling process, for this reason there is a greatly increase of the homogeneity of the powder and the particles are well-dispersed without agglomeration, which is a desirable feature for practical applications. The LDM process generates a limited formation of defects at the particles surface, as shown in Fig. 7b and c. These defects are caused by low-energy impacts produced during the milling process. The micrograph with higher magnifications, Fig. 7d shows the particles presence with size ∼200 nm and a crystallite size in the order 100 nm, that are in agreement with the results calculated from XRD patterns. It is remarkable that this process allows to reduce the particle size avoiding a noticeable amorphization, as shown in Fig. 7d, because milling is carried out here without any liquid media and due to the less energy of the process.
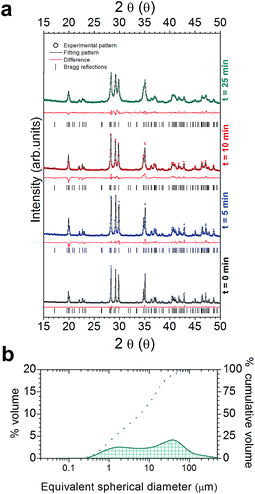
Refinement technique has been applied to gain the structural information about the phase behaviour of the samples milled at different times. The refined data of the samples, based on the figures of merit are compiled in Table 1, Rwp-factor (R-weighted pattern) and χ2 (“Goodness of fit indicator”) and R(F2); the refining converges. In addition, visual results of the plots show the accomplishment of the rietveld refinement, as reflected by small differences between calculated and observed.
| Samples | a (Å) | b (Å) | c (Å) | β (°) | γ (°) | α (°) | Rwp (%) | χ2 | R(F2) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Initial powder | 8.4377(1) | 8.8134(1) | 5.1556(1) | 90 | 93.398(2) | 90 | 8.96 | 4.68 | 6.9 |
| 5 min LDM | 8.4470(2) | 8.8237(2) | 5.1608(1) | 90 | 93.389(2) | 90 | 9.35 | 5.27 | 8.4 |
| 10 min LDM | 8.4421(2) | 8.8193(2) | 5.1583(1) | 90 | 93.392(3) | 90 | 9.37 | 5.79 | 7.2 |
| 20 min LDM | 8.4415(3) | 8.8198(4) | 5.1578(3) | 90 | 93.389(3) | 90 | 9.28 | 5.02 | 8.4 |
| 40 min LDM | 8.4423(2) | 8.8195(3) | 5.1580(2) | 90 | 93.38(3) | 90 | 10.04 | 6.14 | 8.4 |
| 5 min HDM | 8.4431(2) | 8.8199(3) | 5.1585(1) | 90 | 93.387(3) | 90 | 9.63 | 5.11 | 7.5 |
| 10 min HDM | 8.4423(4) | 8.8202(4) | 5.1585(2) | 90 | 93.378(4) | 90 | 9.19 | 4.69 | 6.6 |
| 25 min HDM | 8.4459(10) | 8.8209(10) | 5.1603(6) | 90 | 93.358(10) | 90 | 6.49 | 2.38 | 2.3 |
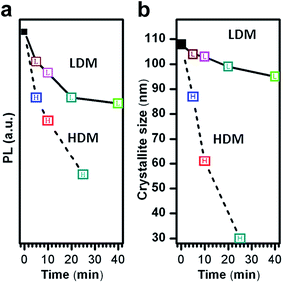
The milling process modified the crystallinity of the powders. To confirm this fact, we have also calculated the crystallite size. The crystallite size of ca. 108 (5), 104 (3), 103 (5), 99 (6) and 95 (5) nm has been obtained for the commercial SrAl2O4:Eu, Dy initial powder and after Low-energy dry milling during 5, 10, 20 and 40 min. The values obtained for the powder were ca. 87(4), 61(5), 30(4) nm after High-energy dry milling (HDM) process during 5, 10 and 25 min.
The photoluminescence intensity and crystallite size as a function of milling time are shown in Fig. 9. The milling process decreases the PL response and the reduction of the intensity is more pronounced by HDM process than by LDM process; indicating that a higher specific surface implies a higher area exposed to surface modifications. This phenomenology is directly related with the reduction of the D because the decreasing of coherent crystalline domain of the material has a greatly influence on the optical properties. The initial crystallite size is ca. 109 nm for SAO powders. The LDM process kept this crystallite size, which suggest that the milling process has low energy to break up the crystallites, as has been reported previously.35 On the contrary, the average crystallite size of the powders from high-energy dry milling has a drastic decrease. It is worth noticing two characteristic aspects, the LDM process has a low energy to break up the crystallites, nevertheless the mean particle size decreases, therefore the decrease of photoluminescence intensity is related with the extrinsic features of the particles. While, the HDM process has a great influence on the intrinsic characteristic of the particles, because the process decrease highly the crystallite size and in this way deteriorates the photoluminescence intensity. It is worth noting that the effect of the parameters in the milling process (rotation speed, milling time, type and size of the container) has a great influence on the final characteristic of the powder. Therefore, the energy transferred by the LDM process is low and enough to reduce the particle size and to avoid the amorphization of the SrAl2O4:Eu, Dy. Thus, the combination of the XRD and FE-SEM allows us to infer that the large variations in PL values by HDM are a consequence of the appearance of the amorphous phase, which provokes an increase in the local chemical heterogeneity of the system and the crystallinity reduction. The lesser variation in PL values by LDM are related to the coherent domain crystalline preservation and the unnoticeable amorphization. And therefore, these results corroborate that the luminescence of the phosphor powders depend extrinsically on the morphology of the particles such as size and intrinsically on the crystallinity.
Generally speaking, the average size of the SAO commercial phosphors is in the range of 20–100 μm which is too large for some applications. Despite the effects of milling on emission intensities, this study presents different approaches that can be selected taking in account the final properties and applications. Low-energy dry milled powders have smooth crystal surfaces while strongly milled powders show rough damaged surfaces and many small fragments. Further work is necessary to determine the efficiency of a mix milling procedure in the processing of phosphors. Probably, an approach that includes a very short high energy dry milling in the first stage and a further low-energy dry milling could be more successful.
4. Conclusions
On the basis of decreasing the particle size by using commercial SrAl2O4:Eu2+, Dy3+ phosphor as raw material, the effects of different milling methods have been evaluated. Wet milling process can significantly alter the structure of the material through hydrolysis even in ethanol media. For overcoming the drawbacks of the wet milling a dry milling-based processes are studied. High energy dry milling process allows a great reduction of the particle size, however milling times above 10 min produces agglomeration and accelerates the decrease of the photoluminescence feature. To solve these issues the low energy dry milling process proposed effectively reduces the particle size to d50 ∼ 2.8 μm, and improves the homogeneity avoiding the amorphization in comparison with previous methods. Regarding the photoluminescent response, its intensity decreases only a 30%, while its average particle size undergoes a decreasing more than 60%. Great break through on the design and reconditioning of this material has been made, taking into account that this purpose is still a critical objective of the phosphor industrial research. In this communication, a special emphasis is placed on the relationship between functional properties and the particles size. It is worth noting that we reported a large phosphorescence ever reported so far by keeping the crystallinity of the particles through low-energy ball-milling.5. Conflict of interest
The authors declare no competing financial interests.Acknowledgements
The authors express their thanks to the MINECO (Spain) project MAT2013-48009-C4-1-P for their financial support. Dr F. Rubio-Marcos is also indebted to MINECO for a “Juan de la Cierva” contract (ref: JCI-2012-14521), which is co-financed with FEDER funds.References
- D. D. M. Van, Tintable luminescent paint, CA Pat., 2309871 C, 2003
.
- Y. Murayama, N. Takeuchi, Y. Aoki and T. Matsuzawa, Phosphorescent phosphor., US Pat., 5424006 A, 1995
.
- F. C. Palilla, A. K. Levine and M. R. Tomkus, J. Electrochem. Soc., 1968, 115, 642–644 CrossRef CAS PubMed
.
- V. Abbruscato, J. Electrochem. Soc., 1971, 118, 930–933 CrossRef CAS PubMed
.
- T. Aitasalo, P. Dereń, J. Hölsä, H. Jungner, J.-C. Krupa, M. Lastusaari, J. Legendziewicz, J. Niittykoski and W. Stręk, J. Solid State Chem., 2003, 171, 114–122 CrossRef CAS
.
- J. Hölsa, H. Jungner, M. Lastusaari and J. Niittykoski, J. Alloys Compd., 2001, 324, 326–330 CrossRef
.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670 CrossRef CAS PubMed
.
- X. Luo, W. Cao and Z. Xiao, J. Alloys Compd., 2006, 416, 250–255 CrossRef CAS PubMed
.
- E. Scimce, Mater. Res. Bull., 1997, 32, 337–341 CrossRef
.
- J. Sanchez-Benitez, A. de Andrés, M. Marchal, E. Cordoncillo, M. V. Regi and P. Escribano, J. Solid State Chem., 2003, 171, 273–277 CrossRef CAS
.
- T. Peng, L. Huajun, H. Yang and C. Yan, Mater. Chem. Phys., 2004, 85, 68–72 CrossRef CAS PubMed
.
- Y. Zhu, M. Zheng, J. Zeng, Y. Xiao and Y. Liu, Mater. Chem. Phys., 2009, 113, 721–726 CrossRef CAS PubMed
.
- S. K. Kandpal, B. Goundie, J. Wright, R. A. Pollock, M. D. Mason and R. W. Meulenberg, ACS Appl. Mater. Interfaces, 2011, 3, 3482–3486 CAS
.
- B. P. Chandra, V. K. Chandra and P. Jha, Solid State Phenom., 2014, 1–65 CrossRef
.
- D. S. Kshatri and a. Khare, Opt. Spectrosc., 2014, 117, 769–783 CrossRef CAS
.
- S. K. Kandpal, B. Goundie, J. Wright, R. A. Pollock, M. D. Mason and R. W. Meulenberg, ACS Appl. Mater. Interfaces, 2011, 3, 3482–3486 CAS
.
- I. Lorite, A. Campo, J. J. Romero and J. F. Fernández, J. Raman Spectrosc., 2012, 43, 889–894 CrossRef CAS PubMed
.
- A. Moure, C. Moure and J. Tartaj, J. Power Sources, 2011, 196, 10543–10549 CrossRef CAS PubMed
.
- V. Šepelák, S. Bégin-Colin and G. Le Caër, Dalton Trans., 2012, 11927 RSC
.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 413–447 RSC
.
- M. Aliofkhazraei, Handbook of Mechanical Nanostructuring, 2015 Search PubMed
.
- M. Sopicka-Lizer, High-Energy Ball Milling, Mechanochemical Processing of Nanopowders, CRC Press, 2010 Search PubMed
.
- R. Von Dreele and A. Larson, General structure analysis system (GSAS), 2004 Search PubMed
.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS
.
- P. Thompson, D. E. Cox and J. B. Hastings, J. Appl. Crystallogr., 1987, 20, 79–83 CrossRef CAS
.
- P. Karen and P. M. Woodward, J. Mater. Chem., 1999, 9, 789–797 RSC
.
- R. A. Young and D. B. Wiles, J. Appl. Crystallogr., 1982, 15, 430–438 CAS
.
- D. Dvoranová, V. Brezová, M. Mazúr and M. a. Malati, Appl. Catal., B, 2002, 37, 91–105 CrossRef
.
- R. A. Schroeder and L. L. Lyons, J. Inorg. Nucl. Chem., 1966, 28, 1155–1163 CrossRef CAS
.
- R. E. Rojas-Hernandez, M. a. Rodriguez and J. F. Fernandez, RSC Adv., 2015, 5, 3104–3112 RSC
.
- A. G. Jones, Crystallization Process Systems, Butterworth-Heinemann, 2002 Search PubMed
.
- E. Cordoncillo, B. Julian-lopez, M. Martínez and M. Luisa, J. Alloys Compd., 2009, 484, 693–697 CrossRef CAS PubMed
.
- H. Zeng, Z. Lin, Q. Zhang, D. Chen, X. Liang, Y. Xu and G. Chen, Mater. Res. Bull., 2011, 46, 319–322 CrossRef CAS PubMed
.
- P. Solsona, S. Doppiu, T. Spassov, S. Suriñach and M. D. Baró, J. Alloys Compd., 2004, 381, 66–71 CrossRef CAS PubMed
.
- R. E. Rojas-Hernandez, M. A. Rodriguez, F. Rubio-Marcos, A. Serrano and J. F. Fernandez, J. Mater. Chem. C, 2015, 3, 1268–1276 RSC
.
| This journal is © The Royal Society of Chemistry 2015 |