Enhanced visible-light photocatalytic performance of BiOBr/UiO-66(Zr) composite for dye degradation with the assistance of UiO-66†
Zhou Shaab and
Jishan Wu*ab
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543. E-mail: chmwuj@nus.edu.sg
bNUS Environmental Research Institute, National University of Singapore, 5A Engineering Drive 1, #02-01, Singapore 117411
First published on 23rd April 2015
Abstract
Because of their many outstanding properties, metal–organic frameworks (MOFs) are considered as a suitable material for various applications in the area of catalysis. Specifically, the superior stability of UiO-66, a zirconium based MOF, makes it a more competitive candidate for the development of a catalyst used in water treatment. In this study, a series of BiOBr/UiO-66 composites with different BiOBr contents were prepared by incorporating UiO-66 with BiOBr through a convenient solution method. In the rhodamine B (RhB) degradation experiment, the resulting BiOBr/UiO-66 composite not only exhibited enhanced photocatalytic activity compared to the control experiment where the mixture of pristine BiOBr and UiO66 was used, but also demonstrated good stability and reusability which was verified by different characterization techniques. The good interaction between UiO-66 and BiOBr was expected to play a crucial role in achieving the excellent properties of the BiOBr/UiO-66 composite. Furthermore, by introducing different scavengers to compete with the potential active species involved in the degradation process, the photocatalytic mechanism of RhB degradation by the BiOBr/UiO-66 composite was investigated.
1. Introduction
Metal–organic frameworks (MOFs) are porous solid materials in which inorganic (metal nodes or metal-containing clusters) and organic units are linked together by strong bonds. The hybridity and flexibility of MOFs gives them many desirable properties, such as high specific surface area, tunable pore size, and relatively good thermostability with exceptional chemical variety. Hence, MOFs have been widely investigated in the areas of gas storage, separation, chemical sensors, catalysis, biomedicine, etc.1–10 In particular, after its first description by Lillerud's group in 2008, a series of zirconium based MOFs (e.g., UiO-66, UiO-67, UiO-68) further expand the potential applications of MOFs, due to their higher thermostability and chemical stability compared to other types of MOFs.11 Among these zirconium based MOFs, UiO-66 (with 1,4-benzenedicarboxylate (BDC) as linker) has been extensively studied for the applications of catalysis, hydrogen generation, gas storage, drug delivery, etc.12–16 More importantly, it is reported that UiO-66 demonstrates high structural stability in water medium,17,18 and such stability can even be preserved after incorporating UiO-66 with active functional groups,19 or introducing missing-linker defects to it.20 Thus, it should be a propitious strategy to incorporate UiO-66 with other functional materials for the applications in aqueous medium, such as water splitting and water treatment.Water contamination by organic pollutants is a serious environmental problem to human society. Among various techniques that have been developed to remove organic pollutants from wastewater, semiconductor based photodegradation is considered as one of the most advantageous strategies since organic pollutants can be directly degraded by this method.21–24 Related to this, visible-light promoted photocatalysis is more attractive due to its efficient utilization of the solar energy.25–27 Based on the superior properties of UiO-66 described earlier, the development of UiO-66 based photocatalyst for visible-light promoted photodegradation can be a promising solution to eliminate organic pollutants from wastewater. Nevertheless, to date, there are only a few reports of photocatalyst developed based on UiO-66 for water treatment.28,29 Therefore, more research efforts are urgently required to develop UiO-66 based visible-light photocatalyst for the effective degradation of organic pollutants in wastewater.
Recently, there was a significant increase in the number of reports focusing on bismuth oxyhalides (BiOX, X = Cl, Br, I) based photocatalysts, owing to their high photocatalytic activity and relatively convenient synthetic procedure.30–33 Among these bismuth oxyhalides, BiOBr is the subject of many studies for visible-light induced photodegradation of organic dyes.34–36 Although pristine BiOBr possesses excellent visible-light photocatalytic activity, there is very limited interspace between the BiOBr particles synthesized without microstructure modulations, leading to the fairly low accessible surface area of this material. Hence, to maximize the potential of BiOBr for photocatalytic application, it is a straightforward method to increase its accessible surface area for more contact of target molecules. To achieve this goal, a common strategy is to modulate the microstructure of BiOBr. However, it is relatively complex to prepare BiOBr with large accessible surface area by controlling its morphology, and surfactant or other mediator is required in the synthetic process.37–39 Alternatively, it is also feasible to enhance the accessible surface area of BiOBr by integrating BiOBr and a supporting material that is sufficiently robust for the photocatalytic process in water medium.
Herein, we report a BiOBr incorporated UiO-66 composite with enhanced visible-light photocatalytic activity for the application in water treatment. This BiOBr/UiO-66 composite is prepared through a convenient solution method, in which the accessible surface area of BiOBr is enlarged and a good interaction is formed between the two components. The photocatalytic activity of the BiOBr/UiO-66 composite was determined by the degradation of rhodamine B (RhB) under visible-light irradiation, and its stability and reusability for dye degradation is evaluated by means of various characterization methods. Furthermore, the photocatalytic mechanism is also studied.
2. Experimental
2.1. Synthesis of UiO-66
The UiO-66 used in this study was synthesized according to a modified procedure reported previously.40 Typically, 80.4 mg ZrCl4 (0.345 mmol) and 57.3 mg terephthalic acid (0.345 mmol) were dissolved in 40 mL N,N′-dimethylformamide (DMF). At the same time, 30 equivalents (with respect to ZrCl4) acetic acid was added as a modulator, and the obtained mixture was stirred at room temperature for 30 min. Thereafter, the mixture was transferred in a 50 mL Teflon-lined autoclave, sealed and heated in an oven at 120 °C for 24 h. The resulting white solid was collected by filtration, thoroughly washed by ethanol, and dried in vacuum oven at 80 °C.2.2. Preparation of BiOBr/UiO-66 composites
BiOBr/UiO-66 composites were prepared through a simple solution method. Five BiOBr/UiO-66 composites with different BiOBr contents were prepared based on the adjustment of the molar ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr. In details, for the preparation of BiOBr/UiO-66 composite with Bi
Zr. In details, for the preparation of BiOBr/UiO-66 composite with Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr molar ratio of (3
Zr molar ratio of (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as BiOBr/UiO-66-3), 113.2 mg NaBr (1.10 mmol) was dissolved in 8 mL HBr aqueous solution (pH was around 2.5). Then, 50 mg UiO-66 (7.7 × 10−3 mmol, formula reported as Zr24O120C192H96 ref. 11) was added, and the mixture was kept stirring vigorously for 1 h. Afterwards, 266.8 mg Bi(NO3)3·5H2O (0.55 mmol) was dissolved in 2 mL DMF, and this Bi(NO3)3 DMF solution was added to the UiO-66 mixture dropwise to reach the total reaction mixture of 10 mL in volume. After stirred for another 30 min, the mixture was kept undisturbed for 3 h at room temperature. Finally, the product was collected by filtration, thoroughly washed with deionized water for several times, and dried in vacuum oven at 80 °C. Other BiOBr/UiO-66 composites with Bi
1) (denoted as BiOBr/UiO-66-3), 113.2 mg NaBr (1.10 mmol) was dissolved in 8 mL HBr aqueous solution (pH was around 2.5). Then, 50 mg UiO-66 (7.7 × 10−3 mmol, formula reported as Zr24O120C192H96 ref. 11) was added, and the mixture was kept stirring vigorously for 1 h. Afterwards, 266.8 mg Bi(NO3)3·5H2O (0.55 mmol) was dissolved in 2 mL DMF, and this Bi(NO3)3 DMF solution was added to the UiO-66 mixture dropwise to reach the total reaction mixture of 10 mL in volume. After stirred for another 30 min, the mixture was kept undisturbed for 3 h at room temperature. Finally, the product was collected by filtration, thoroughly washed with deionized water for several times, and dried in vacuum oven at 80 °C. Other BiOBr/UiO-66 composites with Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr molar ratios of (0.5
Zr molar ratios of (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as BiOBr/UiO-66-0.5), (1
1) (denoted as BiOBr/UiO-66-0.5), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as BiOBr/UiO-66-1), (2
1) (denoted as BiOBr/UiO-66-1), (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as BiOBr/UiO-66-2), and (4
1) (denoted as BiOBr/UiO-66-2), and (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as BiOBr/UiO-66-4) were also prepared following the similar procedure by adjusting the amount of precursors for BiOBr. Pristine BiOBr was also synthesized by the same procedure of BiOBr/UiO-66-3, excluding the addition of UiO-66.
1) (denoted as BiOBr/UiO-66-4) were also prepared following the similar procedure by adjusting the amount of precursors for BiOBr. Pristine BiOBr was also synthesized by the same procedure of BiOBr/UiO-66-3, excluding the addition of UiO-66.
2.3. Characterization
Powder XRD data was collected from a Bruker-AXS D8 DISCOVER with GADDS powder X-ray diffractometer. The Cu Kα line (λ = 1.5406 Å) was used as the radiation source. The product morphology was characterized by electron microscopy (JEOL JSM-6701F FESEM with integrated Oxford EDS system, and JEOL JSM-3010 TEM). UV-Vis diffuse reflectance spectra were recorded by a Shimadzu UV-2450 UV/VIS spectrometer with an ISR-240A Integrating Sphere Attachment, with BaSO4 as reference. The band gap energy of the samples was calculated by the following equation:41| αhν = A(hv − Eg)n/2 |
2.4. Evaluation of the photocatalytic activity
The photocatalytic activity of the catalyst was evaluated by measuring the absorbance of the RhB solution at 554 nm during the degradation process. For the degradation experiment with BiOBr/UiO-66-3, 15 mg sample was mixed with 30 mL RhB aqueous solution (0.03 mM) in a 50 mL round bottom flask. To reach complete adsorption equilibrium, the mixture was first stirred thoroughly with a magnetic stirrer in dark for 1 h. Afterwards, the suspension was irradiated by a 500 W halogen lamp. A 420 nm cutoff filter (Newport, 65CGA-420) and a water filter were placed between the suspension and the light source to eliminate the UV and infrared irradiation. The irradiation intensity in the center of the flask was measured to be about 82 mW cm−2 by an Ophir Nova II power/energy meter. During the entire process of dye degradation, the solid suspension was under magnetic stirring, and to maintain constant dissolved oxygen content, air was continuously bubbled to the reaction mixture at a rate of 10 mL min−1. At certain time intervals, 0.8 mL aliquots were sampled and centrifuged. The dye concentration of the clear supernatant was then measured by a Shimadzu UV-1700 UV/Vis spectrometer. For the degradation experiment with BiOBr/UiO-66-0.5, BiOBr/UiO-66-1, BiOBr/UiO-66-2, and BiOBr/UiO-66-4, as well as the control experiments, all conditions were the same as those in the experiment with BiOBr/UiO-66-3. Three control experiments were conducted separately with different catalysts added to the RhB solution, which were the mechanical mixture of pristine UiO-66 and BiOBr, pristine UiO-66, and pristine BiOBr; the amount of pristine UiO-66 or BiOBr was equal to the actual amount of that in BiOBr/UiO-66-3. The blank experiment was also handled under the same conditions, but no catalyst was added.2.5. Investigation of the photocatalytic mechanism
To study the photocatalytic mechanism of the catalyst, isopropyl alcohol (IPA), benzoquinone (BQ), and EDTA were introduced as scavengers for hydroxyl radicals (HO˙), superoxide radicals (O2−˙), and holes (h+), respectively.44–46 IPA, BQ, or EDTA was added to the RhB solution to obtain concentrations of 500 mM, 1 mM, or 1 mM before the addition of BiOBr/UiO-66-3, and all other conditions were remained the same as those used in the photodegradation experiment. For the experiment with N2 purging, air was replaced by N2 to bubble in the suspension at the same rate with other conditions unchanged. The potential HO˙ formed on the surface of the BiOBr/UiO-66-3 composite was detected by the photoluminescence technique, in which terephthalic acid was used as a probe molecule.47 The detection process was similar to the evaluation of photocatalytic activity, except that RhB solution was replaced by 30 mL aqueous solution of 5 × 10−4 M terephthalic acid and 2 × 10−3 M NaOH. During light irradiation, 0.8 mL reaction solution was sampled at selected timing to measure the fluorescence intensity at 425 nm by a Shimadzu RF-5301 PC fluorescence spectrometer with the excitation wavelength of 315 nm.3. Results and discussion
3.1. Material characterization
The composition of different samples was characterized by XRD. As shown in Fig. 1a, the XRD diffraction pattern of pristine UiO-66 is in line with the pattern described in literature, indicating the successful synthesis of UiO-66 in this study.11 All the diffraction peaks of pristine BiOBr shown in Fig. 1b can be assigned to the tetragonal BiOBr phase (JCPDS: 73-2061), and these peaks can also be observed in the XRD diffraction patterns of BiOBr/UiO-66-3 (Fig. 1b) and other BiOBr/UiO-66 composites (Fig. S1†). Several strong diffraction peaks from UiO-66 are discernable in BiOBr/UiO-66-3 (indicated by arrows in Fig. 1b), suggesting that the structure of UiO-66 remains undamaged under the conditions of BiOBr synthesis. Although the strength of the diffraction peaks of UiO-66 gets weaker with the decrease in the content of UiO-66 in the BiOBr/UiO-66 composites, the two peaks between 6° and 10° can be observed readily even in the pattern of BiOBr/UiO-66-4, which possesses the highest BiOBr and lowest UiO-66 content in this study (Fig. S1†). Interestingly, compared to the XRD pattern of pristine BiOBr (Fig. 1b), there is an intensity change in the peaks belonging to different facets of BiOBr that is as a component of the composite, especially in terms of the (001), (102) and (110) facets. Thus, it is expected that UiO-66 may affect the growth process of BiOBr crystals, leading to different growing preference of the crystal facets. | ||
| Fig. 1 XRD patterns of (a) pristine UiO-66; (b) BiOBr/UiO-66-3 (i), BiOBr/UiO-66-3 after RhB degradation experiment (ii), and pristine BiOBr(iii). | ||
Fig. 2a and b show the SEM images of pristine UiO-66. The pristine UiO-66 appears as relatively uniform particles, and the size of these particles are less than 200 nm. As shown in Fig. 2c and d, after the incorporation of BiOBr, the BiOBr/UiO-66-3 sample reveals some feature of a hybrid composite that particles and flakes are combined together evenly. The particles should be UiO-66, which also supports the XRD result that UiO-66 is stable during the synthesis of BiOBr. The flakes inserted among the UiO-66 particles are believed to be BiOBr, and BiOBr with similar morphology has also been described in previous studies.48,49 The dimension of the BiOBr flakes in BiOBr/UiO-66-3 is around a few hundred nanometers and their thickness is less than 50 nm. It is also discernable that these BiOBr flakes arrange randomly, and their interspace is visibly increased due to the presence of UiO-66 particles. In addition, from the EDS mapping images of BiOBr/UiO-66-3, Zr, Bi and Br elements are found evenly distributing in the composite (Fig. S2†), further confirming the uniform combination of UiO-66 and BiOBr to form the composite. Although pristine BiOBr also presents similar morphology, their arrangement is in a more orderly pattern. As shown in Fig. S3,† the flakes in pristine BiOBr pack together in a more compact manner than that in the BiOBr/UiO-66 composite, thus pristine BiOBr is expected to possess smaller accessible interspace.
The morphology of these samples was also characterized by TEM. It can be seen from Fig. 3a and b that most of the UiO-66 particles are around 100 nm in size. For BiOBr/UiO-66-3, the UiO-66 particles are still clearly identifiable, in agreement with the results of XRD and SEM (Fig. 2c and d). Among the UiO-66 particles, the BiOBr flakes can also be observed. Although the exact size of the BiOBr flakes is difficult to estimate from the TEM images, the thickness of the flakes is within the range of 10 to 20 nm. Besides, pristine BiOBr flakes are about 200–400 nm in size with a rounded edge, and they also exhibit a compact pattern, as shown in the TEM image (Fig. S4†).
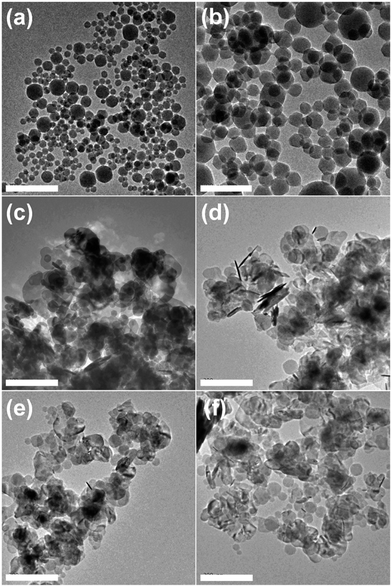 | ||
| Fig. 3 TEM images of (a and b) pristine UiO-66, (c and d) BiOBr/UiO-66-3, and (e and f) BiOBr/UiO-66-3 after RhB degradation experiment (scale bars are 200 nm in (b), and 500 nm in other images). | ||
The specific surface areas of the BiOBr/UiO-66 composites, pristine UiO-66 and pristine BiOBr were evaluated by N2 adsorption (Table 1). And the plots of adsorption–desorption isotherms and pore size distributions are shown in Fig. S5.† As shown in Table 1, the specific surface area of pristine UiO-66 is 869 m2 g−1. Because the synthesis conditions were not optimized, this value is slightly lower than that of some model examples, such as 1069 m2 g−1 and 1110 m2 g−1,42,50 but it is comparable to the value reported by other studies, e.g., 850 m2 g−1 or 931 m2 g−1.51,52 After the incorporation of BiOBr, the specific surface areas of the BiOBr/UiO-66 composites remarkably decrease with the enhancement of BiOBr contents. This is not an unexpected result, since the specific surface area of pristine BiOBr is only 14.8 m2 g−1, about 58-fold smaller compared to that of pristine UiO-66. Similarly, because of the smaller total pore volume of pristine BiOBr than that of pristine UiO-66, the same trend of the total pore volumes of BiOBr/UiO-66 composites decreasing with the increase in BiOBr content is also observed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio, specific surface area and total pore volume) and photocatalytic activity (reaction rate constant) of different catalyst samples
Zr ratio, specific surface area and total pore volume) and photocatalytic activity (reaction rate constant) of different catalyst samples
| Sample | Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratioa Zr ratioa |
Specific surface areab/m2 g−1 | Total pore volumec/cm3 g−1 | Reaction rate constant (k)d/min−1 |
|---|---|---|---|---|
a The Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratios were molar ratios, and were determined based on the reaction precursor.b The specific surface areas were evaluated by the BET method.c The total pore volumes were obtained at P/P0 = 0.99.d The reaction rate constants (k) were calculated based on a pseudo-first-order kinetic model.e The reaction rate constant was obtained from the control experiment in which only pristine UiO-66 was used, and the amount of UiO-66 was equal to the actual amount of that contained in BiOBr/UiO-66-3.f The reaction rate constant was obtained from the control experiment in which only pristine BiOBr was used, and the amount of BiOBr was equal to the actual amount of that contained in BiOBr/UiO-66-3.g The reaction rate constant was obtained from the control experiment in which the mixture of pristine UiO-66 and BiOBr was used, and the amount of UiO-66 or BiOBr was equal to the actual amount of that contained in BiOBr/UiO-66-3. Zr ratios were molar ratios, and were determined based on the reaction precursor.b The specific surface areas were evaluated by the BET method.c The total pore volumes were obtained at P/P0 = 0.99.d The reaction rate constants (k) were calculated based on a pseudo-first-order kinetic model.e The reaction rate constant was obtained from the control experiment in which only pristine UiO-66 was used, and the amount of UiO-66 was equal to the actual amount of that contained in BiOBr/UiO-66-3.f The reaction rate constant was obtained from the control experiment in which only pristine BiOBr was used, and the amount of BiOBr was equal to the actual amount of that contained in BiOBr/UiO-66-3.g The reaction rate constant was obtained from the control experiment in which the mixture of pristine UiO-66 and BiOBr was used, and the amount of UiO-66 or BiOBr was equal to the actual amount of that contained in BiOBr/UiO-66-3. |
||||
| BiOBr/UiO-66-0.5 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
486 | 0.75 | 0.1815 |
| BiOBr/UiO-66-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
382 | 0.61 | 0.2261 |
| BiOBr/UiO-66-2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
278 | 0.55 | 0.2508 |
| BiOBr/UiO-66-3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
204 | 0.36 | 0.2554 |
| BiOBr/UiO-66-4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
149 | 0.29 | 0.2179 |
| Pristine UiO-66 | — | 869 | 1.19 | 0.0044e |
| Pristine BiOBr | — | 14.8 | 0.12 | 0.1206f |
| Control (UiO-66 & BiOBr mixture) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 0.1333g |
The optical absorption abilities of the samples at different wavelengths were investigated by UV-Vis diffuse reflectance measurement. As illustrated in Fig. 4a, pristine UiO-66 is transparent in the range of 340 to 800 nm, inferring that UiO-66 can only absorb UV-light. A similar absorption edge at about 440 nm is observed in pristine BiOBr and all the BiOBr/UiO-66 composites, demonstrating that their optical absorption extends to the visible-light range. In addition, the band gap energies (Eg) of these samples can be extrapolated from the spectra. According to Fig. 4b, the Eg of pristine UiO-66 and BiOBr are about 4.0 eV and 2.8 eV, in line with the reported results.53,54 Furthermore, the Eg of all the BiOBr/UiO-66 composites closely distribute in the range between 2.8 eV and 2.9 eV, implying their potential ability to utilize the visible-light energy. Fig. 4a shows that the absorption intensities of BiOBr/UiO-66 composites gradually decrease with the increase of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratios in the range of 200 to about 370 nm. The absorption in this range should be attributed to the absorption of the organic linkers in UiO-66 as well as that of the junction part between BiOBr and UiO-66.14,29 So it is rational to infer that with the increase of Bi
Zr ratios in the range of 200 to about 370 nm. The absorption in this range should be attributed to the absorption of the organic linkers in UiO-66 as well as that of the junction part between BiOBr and UiO-66.14,29 So it is rational to infer that with the increase of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio, the amount of UiO-66 in the BiOBr/UiO-66 composite decreases, leading to the decline of absorption intensity between 200 and 370 nm.
Zr ratio, the amount of UiO-66 in the BiOBr/UiO-66 composite decreases, leading to the decline of absorption intensity between 200 and 370 nm.
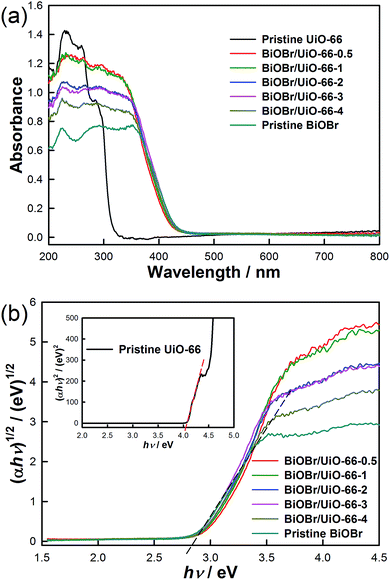 | ||
| Fig. 4 (a) UV-Vis diffuse reflectance spectra, and (b) plots of (αhν)1/2 (insert is (αhν)2) versus energy (hν) to obtain the band gap energies of the samples. | ||
3.2. Photocatalytic activity
To evaluate the photocatalytic activity of different samples, the degradation of RhB by these samples in aqueous solution under visible-light irradiation was examined. As illustrated in Fig. 5a, all the BiOBr/UiO-66 composites show remarkably high activity, as nearly all RhB molecules could be completely degraded in merely 15 min. However, it is not easy to distinguish the activity order of different BiOBr/UiO-66 composites. Thus, by assuming that the reactions follow a pseudo-first-order kinetic model, the reaction rate constants (k) were calculated to quantify the degradation rates of different experiments (Fig. 5b and Table 1). Based on the comparison of reaction rate constants, it is not difficult to find that BiOBr/UiO-66-3 exhibits the fastest degradation rate and as such, the highest photocatalytic activity. Furthermore, when the degradation experiment of BiOBr/UiO-66-3 is extended to 30 min, the intermediate degradation products of RhB can be further degraded, and no distinguishable absorption peak in the range of 300–700 nm can be observed (Fig. S6†). For the control experiment with the mechanical mixture of pristine UiO-66 and BiOBr, although the components are the same as those in the BiOBr/UiO-66-3 sample, its photocatalytic activity is much lower than that of any BiOBr/UiO-66 composite. The similar result can also be observed from the control experiment in which pristine BiOBr is used. Besides, if pristine UiO-66 is introduced in the degradation experiment, only slight decrease in RhB concentration can be observed. As described earlier, pristine UiO-66 does not possess visible-light activity, it is inferred that this tiny concentration drop is attributed to the weak physical adsorption after the equilibrium. Besides, the result of the blank experiment reveals that the photolysis of RhB without the addition of any catalyst is trifling (Fig. 5a, blank).Since the photocatalytic activity of BiOBr/UiO-66-3 is much higher than that of the mechanical mixture with the same components, a potential interaction is anticipated to form between UiO-66 and BiOBr in the BiOBr/UiO-66 composite. As described previously, the morphology of pristine BiOBr shows a compact pattern and the BiOBr flakes pack together so that the accessible interspace between the flakes is very limited. In contrast, in the presence of UiO-66, the synthesized BiOBr flakes expose in a more random manner, which may be resulted from two reasons. First, the UiO-66 particles uniformly distribute and insert between the BiOBr flakes, preventing the overlap of these flakes. Another possible reason is the potential interaction between UiO-66 and BiOBr, through which the growth of BiOBr can be affected by UiO-66. Due to the random distribution of the UiO-66 particles, the growth of BiOBr crystals also exhibits such random behavior. As a result, both effects give rise to the random arrangement of BiOBr flakes in the BiOBr/UiO-66 composite, increasing the accessible interspace in the composite. Therefore, compared to pristine BiOBr, the BiOBr/UiO-66 composites have more accessible BiOBr surface to contact with RhB molecules, leading to the enhancement of RhB degradation rate. In addition, the content of BiOBr in the BiOBr/UiO-66 composite is another factor to be considered. From the reaction rate constants summarized in Table 1, the photocatalytic activity of the BiOBr/UiO-66 composite rises with the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio increasing from (0.5
Zr ratio increasing from (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to (3
1) to (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It is obvious that the increased BiOBr content results in the increased active sites of BiOBr in the BiOBr/UiO-66 composite, so that the RhB degradation rate is enhanced. However, when the Bi
1). It is obvious that the increased BiOBr content results in the increased active sites of BiOBr in the BiOBr/UiO-66 composite, so that the RhB degradation rate is enhanced. However, when the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio is further increased to (4
Zr ratio is further increased to (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the photocatalytic activity of the composite apparently drops to the level even lower than that of the composite with (1
1), the photocatalytic activity of the composite apparently drops to the level even lower than that of the composite with (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) Bi
1) Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio. A possible explanation is that when excessive BiOBr is incorporated to the BiOBr/UiO-66 composite, the amount of UiO-66 may not be enough to separate most of the BiOBr flakes, and therefore the growth of the excessive BiOBr could not be affected. As a result, the behavior of the exceeding BiOBr should be more similar to that of pristine BiOBr, tending to aggregate on the composite surface. This may reduce the accessible surface area of BiOBr and also block the potential channels formed in the final BiOBr/UiO-66 composite, through which RhB molecules can contact with more exposing surface of BiOBr. Consequently, exceeding BiOBr content may reduce the accessible surface area of BiOBr, and have negative effect on the photocatalytic activity of the BiOBr/UiO-66 composite. Therefore, to obtain the highest photocatalytic activity in this study, the optimum BiOBr content in the BiOBr/UiO-66 composite is obtained when Bi
Zr ratio. A possible explanation is that when excessive BiOBr is incorporated to the BiOBr/UiO-66 composite, the amount of UiO-66 may not be enough to separate most of the BiOBr flakes, and therefore the growth of the excessive BiOBr could not be affected. As a result, the behavior of the exceeding BiOBr should be more similar to that of pristine BiOBr, tending to aggregate on the composite surface. This may reduce the accessible surface area of BiOBr and also block the potential channels formed in the final BiOBr/UiO-66 composite, through which RhB molecules can contact with more exposing surface of BiOBr. Consequently, exceeding BiOBr content may reduce the accessible surface area of BiOBr, and have negative effect on the photocatalytic activity of the BiOBr/UiO-66 composite. Therefore, to obtain the highest photocatalytic activity in this study, the optimum BiOBr content in the BiOBr/UiO-66 composite is obtained when Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr ratio equals (3
Zr ratio equals (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
To demonstrate the benefit of using UiO-66, the photocatalytic performance of BiOBr/UiO-66-3 was compared with that of BiOBr incorporated with NH2-modified UiO-66 (UiO-66-NH2) and Fe-MIL-53-NH2 (an iron based MOF), respectively. UiO-66-NH2 was prepared by the same synthesis procedure of UiO-66, except that terephthalic acid was replaced with the same molar amount of 2-aminoterephthalic acid. Fe-MIL-53-NH2 was synthesized according to the reported procedure.55 Then the BiOBr/UiO-66-NH2 and BiOBr/Fe-MIL-53-NH2 composites containing the same amount of BiOBr as that in BiOBr/UiO-66-3 (denoted as BiOBr/UiO-66-NH2-3 and BiOBr/Fe-MIL-53-NH2-3) were prepared (Fig. S7 and S8†). The results of the RhB degradation experiments indicate that both BiOBr/UiO-66-NH2-3 and BiOBr/Fe-MIL-53-NH2-3 exhibit slower RhB degradation rate than BiOBr/UiO-66-3 (Fig. S9†), which proves the advantage of using UiO-66 in the BiOBr/MOF composite for photocatalytic application.
3.3. Photocatalyst stability
The structural stability of the BiOBr/UiO-66 composite was evaluated by comparing the change in XRD diffraction patterns and morphology before and after the dye degradation experiment. As shown in Fig. 1b, no apparent difference can be found in the XRD patterns of BiOBr/UiO-66-3 used for dye degradation compared to the freshly prepared composite. In addition, from the SEM images, the morphology of BiOBr/UiO-66-3 used for RhB degradation (Fig. 2e and f) almost remains the same as that before the degradation (Fig. 2c and d). The same conclusion can also be drawn based on the TEM results (Fig. 3e and f). The stability and reusability of BiOBr/UiO-66-3 were further examined by reusing the catalyst for two more degradation cycles. As illustrated in Fig. S10,† although the degradation rate drops slightly in the second cycle, most of the dye can still be degraded in 15 min. With regard to the decrease of photocatalytic activity for the recycled catalyst, one possible explanation is that part of the degradation products generated in the first degradation cycle might be adsorbed on the surface or trapped in the porous structure of the composite, causing the reduction in the surface area of BiOBr accessible to the dye molecules in the second cycle.56 However, no further decay in the degradation rate is observed in the third cycle, which proves that BiOBr/UiO-66 composite is a stable photocatalyst for long term degradation process.Apparently, the superior stability of UiO-66 should be an important factor contributing to the remarkable stability of the BiOBr/UiO-66 composite. And the good interaction between UiO-66 and BiOBr may also be beneficial to the stability of the final composite. As interpreted earlier, this interaction may affect the growth process of the BiOBr crystals. In fact, this interaction might also be essential to establish a good combination between UiO-66 particles and the synthesized BiOBr flakes. To achieve this effect, during the preparation process of BiOBr/UiO-66 composites, bromide salt was first introduced in the UiO-66 aqueous suspension. Because the Zr6O4(OH)4 octahedra in the UiO-66 structure (pink clusters shown in Fig. S11†) are positively charged, there should be electrostatic interactions occurring between the octahedra and the negatively charged bromide ions.42 As a result, these bromide ions might accumulate around the UiO-66 frameworks and react with the subsequently added Bi2O22+ cations. Finally, the BiOBr crystal seeds generated initially could attach to the UiO-66 particles, and the BiOBr flakes grown around should form good interaction with UiO-66. To verify this mechanism, the intermediate products generated at 1, 3, 5, 10, and 15 min during the synthesis of BiOBr/UiO-66-0.5 were sampled and examined by TEM. As shown in Fig. S12,† with the increase of synthesis time, more and more BiOBr flakes grow and attach on the surface of the UiO-66 particles. Moreover, the magnified images of the 1 min (Fig. S12b†) and 3 min (Fig. S12d†) samples clearly show that the initially generated BiOBr attaches and wraps on the UiO-66 particles. This result demonstrates the good interaction between the two components in the BiOBr/UiO-66 composite.
3.4. Photocatalytic mechanism
To study the photocatalytic mechanism of the BiOBr/UiO-66 composite for the degradation of RhB, the potential roles of HO˙, O2−˙, and h+ (the three main active species involved in the photocatalytic oxidation process) during the degradation process were investigated.21,57–59 IPA, BQ, and EDTA were separately introduced in the degradation process to attempt to trap HO˙, O2−˙, and h+.44–46 As shown in Fig. 6, the degradation rate is significantly restrained in the presence of BQ, which is able to quench O2−˙, inferring that O2−˙ should be a major contributor to the decomposition of RhB. Considering that the dissolved O2 is the crucial reagent to form O2−˙, instead of air, N2 was purged in the degradation system to study its effect on the degradation rate. Similarly, N2 purging also has a negative effect on the degradation rate, further confirming the important role of O2−˙. However, either the addition of BQ or the N2 purging only partially suppresses the degradation reaction, implying that RhB was also degraded by other routes. Afterwards, IPA was added as a scavenger of HO˙. However, the degradation rate almost does not affected, suggesting that HO˙ is not a dominant active species involved in the degradation process. To further confirm the role of HO˙, a photoluminescence technique was applied, in which terephthalic acid was used as a probe molecule to monitor the HO˙ generated on the surface of the photocatalyst.47 As shown in Fig. S13,† no obvious enhancement in the fluorescence intensity is observable during the test period, further verifying that HO˙ is an unimportant active species for degradation system. To have a further understanding of the photocatalytic mechanism, EDTA was introduced to the system to suppress the photogenerated h+. As illustrated in Fig. 6, the degradation of RhB is almost totally restrained. It is known that EDTA can suppress the activity of h+ and may also accelerate the recombination of e− and h+, hence the formation of O2−˙ was also suppressed in the presence of EDTA. As a result, the degradation of RhB was almost inhibited by EDTA due to the suppressed generation of h+ and O2−˙. Therefore, the photogenerated h+ is also involved in the RhB degradation process. The conclusion that O2−˙ and h+ are the two main active species for the BiOBr based photocatalyst system was also confirmed by other research groups.34,60,61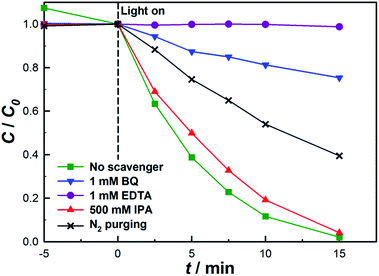 | ||
| Fig. 6 Effects of different scavengers on the degradation of RhB in the presence of BiOBr/UiO-66-3 under visible-light irradiation. | ||
4. Conclusion
In summary, a novel BiOBr/UiO-66 composite was prepared through a convenient solution method for the degradation of RhB under visible-light irradiation. All the BiOBr/UiO-66 composites with different BiOBr contents exhibited excellent photocatalytic activity for the degradation of RhB. Specifically, the composite with Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr molar ratio equaling (3
Zr molar ratio equaling (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could decompose all RhB molecules in 15 min, and also was able to further degrade the intermediate degradation products in another 15 min. Compared to the control experiments in which pristine UiO-66 and/or BiOBr was used, the enhanced photocatalytic activity provided by the BiOBr/UiO-66 composite should be ascribed to the enlarged accessible surface area of BiOBr in the composite, which was achieved with the assistance of UiO-66 and the good interaction between BiOBr and the UiO-66 frameworks. In addition, such good interaction as well as the outstanding stability of UiO-66 is believed to be beneficial to establish the good stability and reusability of the BiOBr/UiO-66 composite in the RhB degradation. The study on the photocatalytic mechanism of the BiOBr/UiO-66 composite implies that O2−˙ and h+ should be the main active species involved in the RhB degradation process. Last but not least, this study is of considerable significance because it opens up numerous opportunities to the development of various MOF based visible-light photocatalysts for water treatment and other related fields in the future.
1) could decompose all RhB molecules in 15 min, and also was able to further degrade the intermediate degradation products in another 15 min. Compared to the control experiments in which pristine UiO-66 and/or BiOBr was used, the enhanced photocatalytic activity provided by the BiOBr/UiO-66 composite should be ascribed to the enlarged accessible surface area of BiOBr in the composite, which was achieved with the assistance of UiO-66 and the good interaction between BiOBr and the UiO-66 frameworks. In addition, such good interaction as well as the outstanding stability of UiO-66 is believed to be beneficial to establish the good stability and reusability of the BiOBr/UiO-66 composite in the RhB degradation. The study on the photocatalytic mechanism of the BiOBr/UiO-66 composite implies that O2−˙ and h+ should be the main active species involved in the RhB degradation process. Last but not least, this study is of considerable significance because it opens up numerous opportunities to the development of various MOF based visible-light photocatalysts for water treatment and other related fields in the future.
Acknowledgements
We acknowledge the financial support from the Singapore-Peking-Oxford Research Enterprise (SPORE), COY-15-EWI-RCFSA/N197-1.Notes and references
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127–1129 CrossRef CAS PubMed.
- Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2011, 112, 869–932 CrossRef PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2011, 112, 1105–1125 CrossRef PubMed.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2011, 112, 1232–1268 CrossRef PubMed.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2011, 112, 1126–1162 CrossRef PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- W. Zhang, G. Lu, C. Cui, Y. Liu, S. Li, W. Yan, C. Xing, Y. R. Chi, Y. Yang and F. Huo, Adv. Mater., 2014, 26, 4056–4060 CrossRef CAS PubMed.
- K. Na, K. M. Choi, O. M. Yaghi and G. A. Somorjai, Nano Lett., 2014, 14, 5979–5983 CrossRef CAS PubMed.
- J. He, J. Wang, Y. Chen, J. Zhang, D. Duan, Y. Wang and Z. Yan, Chem. Commun., 2014, 50, 7063–7066 RSC.
- J. Ren, H. W. Langmi, B. C. North, M. Mathe and D. Bessarabov, Int. J. Hydrogen Energy, 2014, 39, 890–895 CrossRef CAS PubMed.
- X. Zhu, J. Gu, Y. Wang, B. Li, Y. Li, W. Zhao and J. Shi, Chem. Commun., 2014, 50, 8779–8782 RSC.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-g. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 CAS.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- H. Zhang, G. Chen and D. W. Bahnemann, J. Mater. Chem., 2009, 19, 5089–5121 RSC.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Nanoscale, 2013, 5, 8326–8339 RSC.
- L. Shen, W. Wu, R. Liang, R. Lin and L. Wu, Nanoscale, 2013, 5, 9374–9382 RSC.
- L. Shen, R. Liang, M. Luo, F. Jing and L. Wu, Phys. Chem. Chem. Phys., 2015, 17, 117–121 RSC.
- X. Zhang, Z. Ai, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- X. Chang, J. Huang, C. Cheng, Q. Sui, W. Sha, G. Ji, S. Deng and G. Yu, Catal. Commun., 2010, 11, 460–464 CrossRef CAS PubMed.
- L. Chen, R. Huang, M. Xiong, Q. Yuan, J. He, J. Jia, M.-Y. Yao, S.-L. Luo, C.-T. Au and S.-F. Yin, Inorg. Chem., 2013, 52, 11118–11125 CrossRef CAS PubMed.
- H. Cheng, B. Huang and Y. Dai, Nanoscale, 2014, 6, 2009–2026 RSC.
- X. Tu, S. Luo, G. Chen and J. Li, Chem.–Eur. J., 2012, 18, 14359–14366 CrossRef CAS PubMed.
- X.-X. Wei, C.-M. Chen, S.-Q. Guo, F. Guo, X.-M. Li, X.-X. Wang, H.-T. Cui, L.-F. Zhao and W. Li, J. Mater. Chem. A, 2014, 2, 4667–4675 CAS.
- H. Li, J. Liu, X. Liang, W. Hou and X. Tao, J. Mater. Chem. A, 2014, 2, 8926–8932 CAS.
- J. Zhang, F. Shi, J. Lin, D. Chen, J. Gao, Z. Huang, X. Ding and C. Tang, Chem. Mater., 2008, 20, 2937–2941 CrossRef CAS.
- H. Cheng, B. Huang, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem.–Eur. J., 2011, 17, 8039–8043 CrossRef CAS PubMed.
- Y. Huo, J. Zhang, M. Miao and Y. Jin, Appl. Catal., B, 2012, 111–112, 334–341 CrossRef CAS PubMed.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- M. A. Butler, J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- W. L. Huang and Q. Zhu, J. Comput. Chem., 2009, 30, 183–190 CrossRef CAS PubMed.
- C. Shifu, J. Lei, T. Wenming and F. Xianliang, Dalton Trans., 2013, 10759–10768 RSC.
- T. B. Li, G. Chen, C. Zhou, Z. Y. Shen, R. C. Jin and J. X. Sun, Dalton Trans., 2011, 6751–6758 RSC.
- S. Shenawi-Khalil, V. Uvarov, S. Fronton, I. Popov and Y. Sasson, Appl. Catal., B, 2012, 117–118, 148–155 CrossRef CAS PubMed.
- K.-i. Ishibashi, A. Fujishima, T. Watanabe and K. Hashimoto, Electrochem. Commun., 2000, 2, 207–210 CrossRef CAS.
- D. Zhang, J. Li, Q. Wang and Q. Wu, J. Mater. Chem. A, 2013, 1, 8622–8629 CAS.
- H. Zhang, Y. Yang, Z. Zhou, Y. Zhao and L. Liu, J. Phys. Chem. C, 2014, 118, 14662–14669 CAS.
- S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700–7702 RSC.
- S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynska, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC.
- X. Zhang, Q. Han and M. Ding, RSC Adv., 2015, 5, 1043–1050 RSC.
- E. Flage-Larsen, A. Røyset, J. H. Cavka and K. Thorshaug, J. Phys. Chem. C, 2013, 117, 20610–20616 CAS.
- M. Shang, W. Wang and L. Zhang, J. Hazard. Mater., 2009, 167, 803–809 CrossRef CAS PubMed.
- S. Bauer, C. Serre, T. Devic, P. Horcajada, J. Marrot, G. Férey and N. Stock, Inorg. Chem., 2008, 47, 7568–7576 CrossRef CAS PubMed.
- S. Song, F. Hong, Z. He, Q. Cai and J. Chen, J. Colloid Interface Sci., 2012, 378, 159–166 CrossRef CAS PubMed.
- M. Pera-Titus, V. Garcıía-Molina, M. A. Baños, J. Giménez and S. Esplugas, Appl. Catal., B, 2004, 47, 219–256 CrossRef CAS PubMed.
- M. D. Hernandez-Alonso, F. Fresno, S. Suarez and J. M. Coronado, Energy Environ. Sci., 2009, 2, 1231–1257 CAS.
- K. Rajeshwar, M. E. Osugi, W. Chanmanee, C. R. Chenthamarakshan, M. V. B. Zanoni, P. Kajitvichyanukul and R. Krishnan-Ayer, J. Photochem. Photobiol., C, 2008, 9, 171–192 CrossRef CAS PubMed.
- J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Catal. Commun., 2011, 13, 63–68 CrossRef CAS PubMed.
- X.-X. Wei, H. Cui, S. Guo, L. Zhao and W. Li, J. Hazard. Mater., 2013, 263(2), 650–658 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XRD figures, SEM and TEM images, EDS images, N2 adsorption–desorption isotherms and pore size distribution figures, UV-Vis spectral data, comparison of dye degradation performance of different BiOBr/MOF composites, data of three degradation cycles, crystal structural illustration of UiO-66, photoluminescence spectra for HO˙ detection. See DOI: 10.1039/c5ra04869a |
| This journal is © The Royal Society of Chemistry 2015 |


