Cubane-type {M4O4} (M = CoII, ZnII, CuII) clusters: synthesis, crystal structures, and luminescent and magnetic properties†
Qian Gaoa,
Yaru Qina,
Yanmei Chena,
Wei Liua,
Haiyan Lia,
Bing Wua,
Yahong Li*a and
Wu Lib
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: liyahong@suda.edu.cn
bQinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China
First published on 6th May 2015
Abstract
The employment of the hydroxyl-rich ligand (E)-3-((2-hydroxy-3-methoxybenzylidene)amino)propane-1,2-diol (H3L) in the chemistry of cubane-type {M4O4} clusters is reported. Three cubane-type clusters of the formula [M4(HL)4] (M = CoII (1), ZnII (2), CuII (3)) were achieved by reactions of metal acetates with H3L under solvothermal conditions. The structures of 1, 2 and 3 have been established by single-crystal X-ray diffraction studies. The tetranuclear clusters 1–3 have cubane-type [M4(μ3-OR)4]4+ cores with divalent metal atoms and deprotonated oxygen atoms (originating from the HL2− ligands) occupying alternate vertices. The luminescence studies suggest strong emission for 2 in the solid state at room temperature. The magnetic properties of 1 and 3 have been investigated. The variable-temperature dc magnetic susceptibility studies indicate ferromagnetic MII⋯MII exchange interactions for 1 and 3. The ac magnetic susceptibility investigation reveals that complex 1 shows slow magnetic relaxation (SMM) behavior.
Introduction
The chemistry of high-nuclearity 3d-metal clusters has received increasing attention. The interest in these clusters spans from pure academic aspects of chemistry to potential applications as functional materials in biological systems,1 magnetism2 and catalysis.3 Among them, cubane-type {M4O4} clusters have attracted a renewed interest. The driving forces for this interest include: (i) the desire to discover new Co4O4-type photocatalysts for water oxidation;4 (ii) efforts to construct M4O4-based single molecule magnets,5 and (iii) requests to develop catalysts for bioinorganic systems and organic synthesis.6 Ligands used in the synthesis of cubane-type {M4O4} clusters can be classified into two main classes: (i) hydroxyl incorporated pyridyl, pyrazole, imidazol or benzimidazol ligands and their derivatives7 and (ii) hydroxyl-rich Schiff base ligands and their reduction products.8 These ligands can function in both bridging and chelating capacities, aggregating metal ions in a cubane-type system.Recently, we have reported the synthesis and ferromagnetic properties of several cubane-type {Ni4O4} clusters supported by a variety of Schiff-base ligands.9 In order to expand the scopes of cubane-type {M4O4} clusters and explore their useful properties, we turned our attention to prepare cubane-type {Co4O4}, {Zn4O4} and {Cu4O4} clusters by employing another Schiff base (E)-3-((2-hydroxy-3-methoxybenzylidene)amino)propane-1,2-diol (H3L, Scheme 1) as a ligand. The H3L ligand has previously been incorporated into trinuclear10 and polynuclear11 manganese complexes, nanoscale multiferroic manganese clusters,12 trinuclear CoII/CoIII mixed-valence complexes13 and Ln4III clusters.14 To our surprise, no cubane-type {M4O4} clusters supported by the H3L ligand were reported. Herein, we present the synthesis, crystal structures, and photoluminescent and magnetic properties of three cubane-type clusters with formula [M(HL)]4 (M = CoII (1), ZnII (2), and CuII (3)). To the best of our knowledge, complex 1 is the first reported cubane-type {Co4O4} cluster supported by Schiff base ligand.
Experimental section
General procedure
X-ray diffraction crystallography
Data were collected at 223(2) K on Rigaku Mercury CCD X-ray diffractometer for 1 and at room temperature on Bruker Smart Apex II diffractometer for 2 and 3 utilizing Mo Kα radiation (λ = 0.71073 Å); the ω and φ scan technique was applied. The structures were solved by direct methods using SHELXS-9715 and refined on F2 using full-matrix least-squares with SHELXL-97.16 Crystallographic data together with refinement details for the new complexes reported in this work are summarized in Table 1. Selected bond lengths and angles for 1–3 are given in the ESI.†| 1 | 2 | 3 | |
|---|---|---|---|
| a Mo Kα radiation.b R1 = ∑(|Fo| − |Fc|)/∑(|Fo|) for observed reflections.c w = 1/[σ2(Fo2) + (αP)2 + bP] and P = [max(Fo2,0) + 2Fc2]/3.d wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2 for all data. | |||
| Formula | C44H52Co4N4O16 | C44H52N4O16Zn4 | C44H52Cu4N4O16 |
| M/g mol−1 | 1128.62 | 1154.38 | 1147.06 |
| T/K | 223(2) | 296(2) | 296(2) |
| λa/Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Tetragonal |
| Space group | C2/c | C2/c | P42/n |
| a/Å | 26.429(5) | 26.369(5) | 15.4331(19) |
| b/Å | 8.4637(17) | 8.4456(13) | 15.4331(19) |
| c/Å | 22.736(5) | 22.718(4) | 9.4388(17) |
| α/° | 90.00 | 90.00 | 90.00 |
| β/° | 114.74(3) | 114.642(6) | 90.00 |
| γ/° | 90.00 | 90.00 | 90.00 |
| V/Å3 | 4619.1(16) | 4598.6(14) | 2248.1(6) |
| Z | 4 | 4 | 2 |
| ρc/g cm−3 | 1.623 | 1.667 | 1.694 |
| μ/mm−1 | 1.487 | 2.138 | 1.944 |
| F(000) | 2320 | 2368 | 1176 |
| θ range/° | 3.00–27.43 | 1.70–28.46 | 1.87 to 27.32 |
| Measd/independent | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211/5236 211/5236 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 879/5736 879/5736 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468/2546 468/2546 |
| Rint reflections | 0.0415 | 0.0592 | 0.0424 |
| Obsd reflns [I > 2σ(I)] | 5236 | 5736 | 2546 |
| GOF on F2 | 1.130 | 0.971 | 1.045 |
| R1b | 0.0606 | 0.0440 | 0.0331 |
| wR2c,d | 0.1632 | 0.1052 | 0.0894 |
| (Δρ)max,min/e Å−3 | 1.023, −0.587 | 0.487, −0.641 | 0.521, −0.281 |
Syntheses of the complexes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was sealed in a Pyrex-tube (8 mL). The tube was heated at 80 °C for 4 days under autogenous pressure. Cooling of the resultant solution to room temperature gave dark green acicular crystals. The crystals were collected by filtration, washed with CH3CN/CH3OH (3 mL, v/v = 3
1) was sealed in a Pyrex-tube (8 mL). The tube was heated at 80 °C for 4 days under autogenous pressure. Cooling of the resultant solution to room temperature gave dark green acicular crystals. The crystals were collected by filtration, washed with CH3CN/CH3OH (3 mL, v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and dried in air. Yield: 0.0186 g (65%, based on Cu). Anal. calcd for C44H52Co4N4O16: C, 46.07; H, 4.57; N, 4.88. Found: C, 45.76; H, 4.50; N, 4.73%. Selected IR data (KBr, cm−1): 3385 s, 1637 s, 1443 s, 1221 s, 1043 m, 749 m.
1) and dried in air. Yield: 0.0186 g (65%, based on Cu). Anal. calcd for C44H52Co4N4O16: C, 46.07; H, 4.57; N, 4.88. Found: C, 45.76; H, 4.50; N, 4.73%. Selected IR data (KBr, cm−1): 3385 s, 1637 s, 1443 s, 1221 s, 1043 m, 749 m.The purity of complexes 1–3 is confirmed by a comparison of experimental and simulated PXRD patterns (Fig. S4–S6†). The experimental peaks are in good agreement with those calculated from X-ray single-crystal diffraction data.
Results and discussion
Syntheses and IR spectra
Complexes 1–2 were prepared under solvothermal conditions in CH3OH. However, the similar reaction between Cu(CH3COO)2·H2O and H3L in CH3OH could not afford any crystalline complex. When the reaction of Cu(CH3COO)2·H2O and H3L was conducted in CH3CN/CH3OH (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a crystalline tetranuclear species [Cu4(HL)4] (3) was generated. The mixed MeCN–MeOH solvent mixture was necessary to ensure adequate solubility of all reagents. Furthermore, when EtOH was used, a lot of dark green copper precipitates were obtained, and no clean product could be isolated from the filtrate.
1), a crystalline tetranuclear species [Cu4(HL)4] (3) was generated. The mixed MeCN–MeOH solvent mixture was necessary to ensure adequate solubility of all reagents. Furthermore, when EtOH was used, a lot of dark green copper precipitates were obtained, and no clean product could be isolated from the filtrate.
The IR spectra of all the complexes show broad peaks in the range of 3346–3385 cm−1 because of the alcoholic OH group. The strong absorption band occurring at 1620, 1631 and 1637 cm−1 for 1, 2 and 3, respectively, can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency of the coordinated ligands, whereas the same band is observed at ca. 1644 cm−1 for the free ligand. The shift of this band towards lower frequency on complexation with the metals suggests coordination via imino nitrogen atom in all the complexes.17 The ν(C–Ophen) mode is present as a very strong band at about 1211–1221 cm−1. The peaks in the range 1043–1082 cm−1 are assigned to alcoholic C–O stretches. Several weak peaks observed for the complexes in the range 3000–2829 cm−1 are ascribed to the aromatic and aliphatic C–H stretches.
N stretching frequency of the coordinated ligands, whereas the same band is observed at ca. 1644 cm−1 for the free ligand. The shift of this band towards lower frequency on complexation with the metals suggests coordination via imino nitrogen atom in all the complexes.17 The ν(C–Ophen) mode is present as a very strong band at about 1211–1221 cm−1. The peaks in the range 1043–1082 cm−1 are assigned to alcoholic C–O stretches. Several weak peaks observed for the complexes in the range 3000–2829 cm−1 are ascribed to the aromatic and aliphatic C–H stretches.
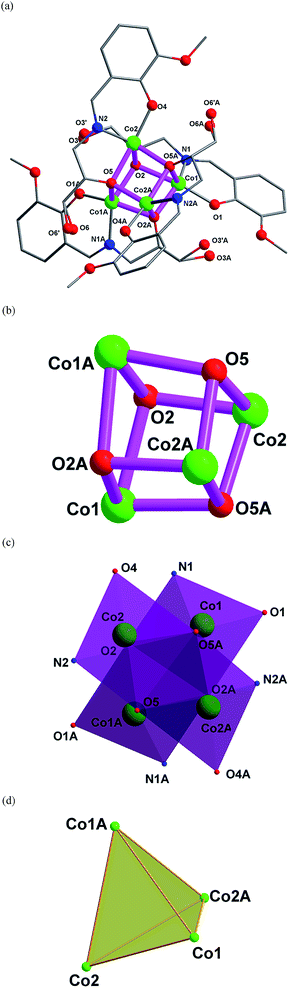
Description of structures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ratio. Four intermolecular hydrogen bonds are formed between two neighboring molecules, resulting in a “wave-like” 1D arrangement of the clusters (Fig. 2).
0.5 ratio. Four intermolecular hydrogen bonds are formed between two neighboring molecules, resulting in a “wave-like” 1D arrangement of the clusters (Fig. 2).
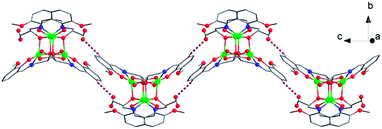 | ||
| Fig. 2 “Wave-like” 1D hydrogen-bonded double chain of 1. Color code: CoII, green; O, red; N, blue; C, gray. | ||
Complex 1 joins a large family of tetranuclear CoII complexes with {Co4O4} cubane cores.4 Among nearly the 140 {Co4O4} clusters found in the Cambridge Structure database, complex 1 is the only one supported by Schiff base, indicating the synthetic novelty of this work.
Complex 2 is one of the members of cubane-type {Zn4O4} family. However, among nearly 100 {Zn4O4} compounds, only limited numbers of complexes were constructed by Schiff base ligands.20
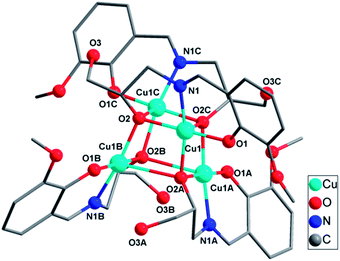 | ||
| Fig. 3 Molecule structure of complex 3; hydrogen atoms have been omitted for clarity. Color code: CuII, light blue; O, red; N, blue; C, gray. | ||
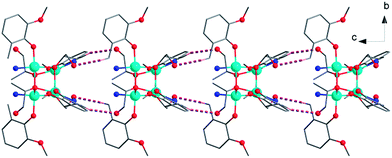 | ||
| Fig. 4 Chain structure formed by hydrogen bonding interactions in 3. Color code: CuII, light blue; O, red; N, blue; C, gray. | ||
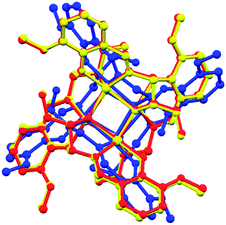
As the {M4O4} cluster of the complexes 1–3 is located about a 2-fold rotation axis. The structures of 1–3 are compared by overlaying the complexes (Fig. 5). The r.m.s values between complexes 1 and 2, 1 and 3, and 2 and 3 are 0.0297, 0.261 and 0.265, respectively, consisting with the space groups of the three complexes.
Luminescence properties
Photoluminescence studies of the ligand H3L and the compounds 1–3 were carried out at room temperature in the solid state (Fig. 6). The free ligand H3L has a moderate fluorescence emission band at 523 nm upon excitation at 370 nm. Complexes 1 and 3 are nonfluorescent and compound 2 has characteristic fluorescence emission. The luminescence of 1 and 3 may be quenched by metal ions. Metal ions can enhance or quench the fluorescence emission of some Schiff base ligands containing aromatic rings, due to magnetic perturbation, redox activity and electronic energy transfer.23 Compound 2 displays a broad emission band at 473 upon excitation at 333 nm. The emission of 2 should be neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal charge transfer (LMCT) in the nature since the ZnII ion is difficult to oxidize or reduce due to its d10 configuration.24 Thus, the luminescent band of 2 can probably be ascribed to intraligand π → π* electron transition.25 The emission intensity of 2 is stronger than that of the ligand. The enhanced fluorescence efficiency of the complex is attributed to the coordination of the ligands to the ZnII ions which effectively increases the rigidity of the ligands and reduces the loss of energy via radiationless thermal vibrations.26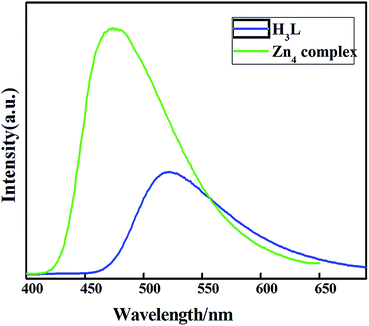 | ||
| Fig. 6 Emission spectra of H3L and 2 (green) in the solid state at room temperature (emission slit = 1 nm). | ||
Magnetic properties of 1 and 3
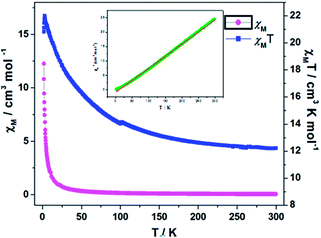
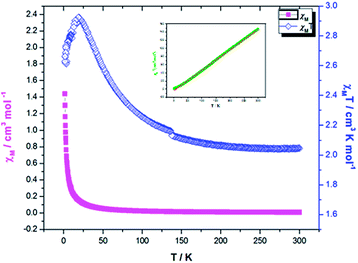
Variable-temperature dc magnetic susceptibility data were recorded for polycrystalline samples of 1 and 3 at an applied magnetic field of 1000 Oe in the temperature range of 2–300 K.The χMT value of 1 at 300 K is 12.22 cm3 K mol−1 (Fig. 7), which is obviously larger than the spin-only value of 7.50 cm3 K mol−1 expected for four S = 3/2 uncoupled spins, possibly due to the orbital contributions of the metal ions.27 Upon cooling from room temperature, χMT per cubane gradually increased to a maximum (21.99 cm3 mol−1 K) at 3 K and then decreased to 20.82 cm3 mol−1 K at 2 K. The observed behavior is indicative of the presence of ferromagnetic exchange interactions. The reciprocal magnetic susceptibilities in 2–300 K follow the Curie–Weiss Law of 1/χM = (T − θ)/C with Curie constant C = 11.95 cm3 K mol−1 and Weiss constant θ = 10.64 K, which confirms the existence of the ferromagnetic interactions among the cubane.
The ac magnetic susceptibility 1 is investigated by measurement at frequencies of 100, 500, 1000, 1250 and 1500 Hz (Fig. 8). Complex 1 shows an unnegligible out-of-phase signal above 2 K in the absence of static field, corresponding to the slow magnetic relaxation in magnetization. Below 10 K the out-of-phase susceptibility (χ′′) shows strong frequency dependence, suggesting this cubane-type {Co4O4} complex is a SMM.
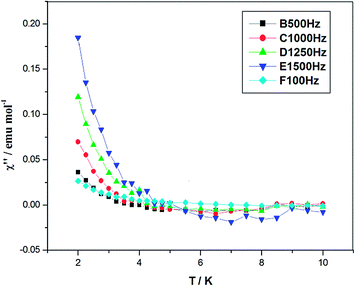 | ||
| Fig. 8 Variable temperature ac susceptibility data at different frequencies without static field for the complex 1. | ||
The value of χMT for 3 is 2.05 cm3 K mol−1 at 300 K (Fig. 9), which is larger than the sum of the expected value (1.50 cm3 K mol−1, g = 2.0, S = 1/2) for four uncoupled CuII ions. As temperature lowered, the χMT value first increases smoothly to reach to a maximum value of 2.80 cm3 K mol−1 at 10 K, and then decrease to minimum value of 2.62 cm3 K mol−1 at 2 K. Fitting the data at 2–300 K with the Curie–Weiss law gives a C of 1.99 cm3 K mol−1 and a θ of 7.99 K. The C value is consistent with the value of 2.05 cm3 K mol−1 at 300 K. The positive value of θ indicates the ferromagnetic couplings among the cubane.
Conclusions
The first use of the hydroxyl-rich ligand (E)-3-((2-hydroxy-3-methoxybenzylidene)amino)propane-1,2-diol (H3L) in the construction of cubane-type {M4O4} clusters was reported. Three new cubane-type complexes [M4(HL)4] (M = CoII (1), ZnII (2), CuII (3)) have been generated. The three complexes described are valuable additions to the chemistry of tetranuclear CoII, ZnII and CuII clusters. Complexes 1 and 2 contain low symmetry cubane cores with different M⋯M distances and M–O–M angles, resulting in three types of {M2O2} faces. Complex 3 belongs to the 4 + 2 class with the four Cu⋯Cu separations being significantly shorter than the other two Cu⋯Cu distances. The photoluminescent studies of 2 indicate the blue shift compared with the H3L ligand, and the emission intensity of the {Zn4O4} complex is stronger than that of the ligand. Magnetic studies for 1 and 3 suggest that complex 1 is a SMM.Acknowledgements
The authors appreciate the financial support from Natural Science Foundation of China (21272167 and 21201127), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institution, and KLSLRC (KLSLRC-KF-13-HX-1).References
- (a) R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239–2314 CrossRef CAS; (b) S. S. Mukhopadhyay, R. J. Staples and W. W. Armstrong, Chem. Commun., 2002, 864–865 RSC; (c) D. V. Zaytsev, V. A. Morozov, J. F. Fan, X. C. Zhu, M. Mukherjee, S. S. Ni, M. A. Kennedy and M. Y. Ogawa, J. Inorg. Biochem., 2013, 119, 1–9 CrossRef CAS PubMed; (d) N. Summa, J. Maigut, R. Puchta and R. Van Eldik, Inorg. Chem., 2007, 46, 2094–2104 CrossRef CAS PubMed.
- (a) M. Manoli, R. Inglis, M. J. Manos, V. Nastopoulos, W. Werndorfer, E. K. Brechin and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2011, 50, 4441–4444 CrossRef CAS PubMed; (b) M. Nakano and H. Oshio, Chem. Soc. Rev., 2011, 40, 3239–3248 RSC; (c) E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146–16155 CrossRef CAS PubMed; (d) G. E. Kostakis, A. M. Ako and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238–2271 RSC; (e) S. Bhattacharya, M. Gnanavel, A. J. Bhattacharyya and S. Natarajan, Cryst. Growth Des., 2014, 14, 310–325 CrossRef CAS; (f) G. E. Kostakis, S. P. Perlepes, V. A. Blatov, D. M. Proserpio and A. K. Powell, Coord. Chem. Rev., 2012, 256, 1246–1278 CrossRef CAS PubMed.
- (a) A. Biswas, L. K. Das, M. G. B. Drew, C. Diaz and A. Ghosh, Inorg. Chem., 2012, 51, 10111–10121 CrossRef CAS PubMed; (b) Q. T. Nguyen and J. H. Jeong, Polyhedron, 2006, 25, 1787–1790 CrossRef CAS PubMed; (c) G. Maayan and G. Christou, Inorg. Chem., 2011, 50, 7015–7021 CrossRef CAS PubMed; (d) S. Thakurta, P. Roy, R. J. Butcher, M. S. E. Fallah, J. Tercero, E. Garribba and S. Mitra, Eur. J. Inorg. Chem., 2009, 4385–4395 CrossRef CAS PubMed; (e) L. J. Wang, C. S. Zhang, Y. H. Xing, Z. Li, N. Xing, L. Y. Wan and H. Shan, Inorg. Chem., 2012, 51, 6517–6528 CrossRef PubMed.
- (a) X. Zhou, F. Li, H. Li, B. B. Zhang, F. S. Yu and L. C. Sun, ChemSusChem, 2014, 7, 2453–2456 CrossRef CAS PubMed; (b) X. B. Han, Z. M. Zhang, T. Zhang, Y. G. Li, W. B. Lin, W. S. You, Z. M. Su and E. B. Wang, J. Am. Chem. Soc., 2014, 136, 5359–5366 CrossRef CAS PubMed; (c) P. F. Smith, C. Kaplan, J. E. Sheats, D. M. Robinson, N. S. McCool, N. Mezle and G. C. Dismukes, Inorg. Chem., 2014, 53, 2113–2121 CrossRef CAS PubMed; (d) F. Evangelisti, R. Guttinger, R. More, S. Luber and G. R. Patzke, J. Am. Chem. Soc., 2013, 135, 18734–18737 CrossRef CAS PubMed; (e) N. S. McCool, D. M. Robinson, J. E. Sheats and G. C. Dismukes, J. Am. Chem. Soc., 2011, 133, 11446–11449 CrossRef CAS PubMed.
- (a) F. J. Klinke, A. Das, S. Demeshko, S. Dechert and F. Meyer, Inorg. Chem., 2014, 53, 2976–2982 CrossRef CAS PubMed; (b) A. Ferguson, J. Lawrence, A. Parkin, J. Sanchez-Benitez, K. V. Kamenev, E. K. Brechin, W. Wolfgang, S. Hill and M. Murrie, Dalton Trans., 2008, 6409–6414 RSC; (c) M. Moragues-Cánovas, M. Helliwell, L. Ricard, É. Rivière, W. Wernsdorfer, E. K. Brechin and T. Mallah, Eur. J. Inorg. Chem., 2004, 2219–2222 CrossRef PubMed; (d) D. N. Hendrickson, E. C. Yang, R. M. Isidro, C. Kirman, J. Lawrence, R. S. Edwards, S. Hill, A. Yamaguchi, H. Ishimoto, W. Wernsdorfer, C. Ramsey, N. Dalal and M. M. Olmstead, Polyhedron, 2005, 24, 2280–2283 CrossRef CAS PubMed.
- (a) S. Thakurta, P. Roy, R. J. Butcher, M. S. El Fallah, J. Tercero, E. Garribba and S. Mitra, Eur. J. Inorg. Chem., 2009, 4385–4395 CrossRef CAS PubMed; (b) J. C. De Paula, W. F. Beck and G. W. Brudvig, J. Am. Chem. Soc., 1986, 108, 4002–4009 CrossRef CAS; (c) Z. B. Zhang, N. Zhao, W. S. Ren, L. Chen, H. B. Song and G. F. Zi, Inorg. Chem. Commun., 2012, 20, 234–237 CrossRef CAS PubMed.
- (a) J. Zhang, P. Teo, R. Pattacini, A. Kermagoret, R. Welter, G. Rogez, T. S. A. Hor and P. Braunstein, Angew. Chem., Int. Ed., 2010, 49, 4443–4446 CrossRef CAS PubMed; (b) A. Das, F. J. Klinke, S. Demeshko, S. Meyer, S. Dechert and F. Meyer, Inorg. Chem., 2012, 51, 8141–8149 CrossRef CAS PubMed; (c) X. Y. Song, Y. H. Xu, L. C. Li, D. Z. Liao and Z. H. Jiang, Inorg. Chim. Acta, 2007, 360, 2039–2044 CrossRef CAS PubMed; (d) Y. L. Zhou, F. Y. Meng, J. Zhang, M. H. Zeng and H. Liang, Cryst. Growth Des., 2009, 9, 1402–1410 CrossRef CAS.
- (a) A. Mukherjee, M. Nethaji and A. R. Chakravarty, Angew. Chem., Int. Ed., 2004, 43, 87–90 CrossRef PubMed; (b) H. Oshio, N. Hoshino and T. Ito, J. Am. Chem. Soc., 2000, 122, 12602–12603 CrossRef CAS; (c) J. M. Clemente-Juan, C. Mackiewicz, M. Verelst, F. Dahan, A. Bousseksou, Y. Sanakis and J. P. Tuchagues, Inorg. Chem., 2002, 41, 1478–1491 CrossRef CAS PubMed; (d) A. B. Canaj, D. I. Tzimopoulos, A. Philippidis, G. E. Kostakis and C. J. Milios, Inorg. Chem., 2012, 51, 10461–10470 CrossRef CAS PubMed.
- (a) S. Y. Zhang, W. Q. Chen, B. Hu, Y. M. Chen, W. Li and Y. H. Li, Inorg. Chem. Commun., 2012, 16, 74–77 CrossRef CAS PubMed; (b) S. Y. Zhang, W. Q. Chen, B. Hu, Y. M. Chen, L. N. Zheng, Y. H. Li and W. Li, J. Coord. Chem., 2012, 65, 4147–4155 CrossRef CAS PubMed; (c) J. W. Ran, S. Y. Zhang, B. Xu, Y. Z. Xia, D. Guo, J. Y. Zhang and Y. H. Li, Inorg. Chem. Commun., 2008, 11, 73–76 CrossRef CAS PubMed.
- S. Nayak, H. P. Nayek, S. Dehnen, A. K. Powell and J. Reedijk, Dalton Trans., 2011, 2699–2702 RSC.
- (a) L. L. Cong, X. T. Qin, W. Sun, Y. Q. Wang, S. Ding and Z. L. Liu, New J. Chem., 2014, 38, 545–551 RSC; (b) P. P. Yang, C. Y. Shao, L. L. Zhu and Y. Xu, Eur. J. Inorg. Chem., 2013, 5288–5296 CrossRef CAS PubMed.
- C. M. Liu, R. G. Xiong, D. Q. Zhang and D. B. Zhu, J. Am. Chem. Soc., 2010, 132, 4044–4045 CrossRef CAS PubMed.
- R. Modak, Y. Sikdar, S. Mandal and S. Goswami, Inorg. Chem. Commun., 2013, 37, 193–196 CrossRef CAS PubMed.
- A. K. Jami, V. Baskar and E. C. Sanudo, Inorg. Chem., 2013, 52, 2432–2438 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures from Diffraction Data, University of Göttingen, Germany, 1997 Search PubMed.
- M. Dolaz, M. Tümer and M. Dığrak, Transition Met. Chem., 2004, 29, 528–536 CrossRef CAS.
- A. W. Addison and T. N. Rao, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- (a) L. L. Hu, Z. Q. Jia, J. Tao, R. B. Huang and L. S. Zheng, Dalton Trans., 2008, 6113–6116 RSC; (b) S. Banerjee, M. Nandy, S. Sen, S. Mandal, G. M. Rosair, A. M. Z. Slawin, C. J. G. Garca, J. M. Clemente-Juan, E. Zangrando, N. Guidolin and S. Mitra, Dalton Trans., 2011, 1652–1661 RSC.
- (a) A. Erxleben, Inorg. Chem., 2001, 40, 208–213 CrossRef CAS PubMed; (b) M. Mikuriya and K. Minowa, Inorg. Chem. Commun., 2000, 3, 227–230 CrossRef CAS.
- A. Mukherjee, R. Raghunathan, M. K. Saha, M. Nethaji, S. Ramasesha and A. R. Chakravarty, Eur. J. Inorg. Chem., 2005, 3087–3096 CAS.
- J. Tercero, E. Ruiz, S. Alvarez, A. Rodríguez-Fortea and P. Alemany, J. Mater. Chem., 2006, 16, 2729–2736 RSC.
- (a) H. F. Zhu, T. A. Okamura, J. Fan, W. Y. Sun and N. Ueyama, New J. Chem., 2004, 28, 1010–1018 RSC; (b) Z. D. Lu, L. L. Wen, Z. P. Ni, Y. Z. Li, H. Z. Zhu and Q. J. Meng, Cryst. Growth Des., 2007, 7, 268–274 CrossRef CAS.
- (a) Q. G. Zhai, X. Y. Wu, S. M. Chen, C. Z. Lu and W. B. Yang, Cryst. Growth Des., 2006, 6, 2126–2135 CrossRef CAS; (b) Z. F. Chen, R. G. Xiong, J. Zhang, X. T. Chen, Z. L. Xue and X. Z. You, Inorg. Chem., 2001, 40, 4075–4077 CrossRef CAS.
- (a) W. J. Niu, J. L. Wang, Y. Bai and B. Dang, Spectrochim. Acta, Part A, 2012, 91, 61–66 CrossRef CAS PubMed; (b) S. Basak, S. Sen, C. Marschner, J. Baumgartner, S. R. Batten, D. R. Turner and S. Mitra, Polyhedron, 2008, 27, 1193–1200 CrossRef CAS PubMed.
- (a) Y. F. Ji, R. Wang, S. Ding, C. F. Du and Z. L. Liu, Inorg. Chem. Commun., 2012, 16, 47–50 CrossRef CAS PubMed; (b) Y. Wang, L. Yi, X. Yang, B. Ding, D. Z. Liao and S. P. Yan, Inorg. Chem., 2006, 45, 5822–5829 CrossRef CAS PubMed.
- (a) A. K. Boudalis, C. P. Raptopoulou, B. Abarca, R. Ballesteros, M. Chadlaoui, J. P. Tuchagues and A. Terzis, Angew. Chem., Int. Ed., 2006, 45, 432–435 CrossRef CAS PubMed; (b) H. Y. Bai, J. F. Ma, J. Yang, Y. Y. Liu, H. Wu and J. C. Ma, Cryst. Growth Des., 2010, 10, 995–1016 CrossRef CAS; (c) C. S. B. Gomes, P. T. Gomes, M. T. Duarte, R. E. D. Paolo, A. L. Macantita and M. J. Calhorda, Inorg. Chem., 2009, 48, 11176–11186 CrossRef CAS PubMed; (d) X. N. Cheng, W. X. Zhang, Y. Z. Zheng and X. M. Chen, Chem. Commun., 2006, 3603–3605 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 996947(1), 996955(2) and 979727(3). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra04868k |
| This journal is © The Royal Society of Chemistry 2015 |