An efficient one pot three-component nanocatalyzed synthesis of spiroheterocycles using TiO2 nanoparticles as a heterogeneous catalyst†
Yogesh Kumar Tailora,
Sarita Khandelwala,
Yogita Kumarib,
Kamlendra Awasthib and
Mahendra Kumar*a
aDepartment of Chemistry, University of Rajasthan, Jaipur, 302004, India. E-mail: mahendrakpathak@gmail.com; Tel: +91-0141-2702720
bSoft Materials Lab, Department of Physics, Malaviya National Institute of Technology, Jaipur, India
First published on 28th April 2015
Abstract
An efficient and environmentally benign isocyanide based domino protocol has been presented for the synthesis of structurally diverse spiroheterocycles, spiroannulated with imidazothiazole and imidazothiadiazole, involving the three component reaction of 2-aminobenzothiazole/2-amino-1,3,4-thiadiazole, cyclohexyl/tert-butyl isocyanides and isatines/cyclic carbonyl compounds catalyzed by recyclable and reusable nanocrystalline TiO2.
Introduction
Modern drug discovery is faced with the challenges of designing chemical reactions that are highly capable of providing target molecules with structural diversity and molecular complexity involving the minimum number of synthetic steps and avoiding the use of volatile organic solvents. The development of efficient and environmentally benign multicomponent domino reactions has attracted increasing interest in modern organic synthesis in view of growing environmental concern. The isocyanide based multicomponent reactions, in particular, combined with eco-compatibility find their significance especially in medicinal chemistry and drug discovery research for the syntheses of drug-like structurally diverse complex molecules in terms of lead finding and lead optimization.1,2 Moreover, the use of low-cost, readily available and reusable catalysts also plays a significant role in organic syntheses and makes the synthetic methods economically-viable. The catalysis by nanoparticles (NPs) seems to be an attractive approach in organic syntheses due to their large reactive surfaces with higher potential for selectivity, which may enable the reactions to proceed efficiently under mild reaction conditions.3 Moreover, the easier isolation of the products and possibility of reusing the catalyst are the additional advantages with the nanocatalyzed multicomponent reactions.4 Recently, TiO2 nanoparticles have emerged as efficient and inexpensive heterogeneous catalyst to promote organic transformations.5 Titanium dioxide (TiO2) catalysis is considered very close to an ideal catalysis because of its sustainability and environmental concerns.6In recent years, the privileged structure–activity concept has been an emerging theme in medicinal chemistry and drug discovery research. Benzothiazole is privileged heterocyclic system and incorporated in marine and terrestrial bioactive natural products in addition to wide range of biopharmaceutical activities of its synthetic derivatives.7,8 Benzothiazole derivatives have been evaluated as potential amyloid-binding diagnostic agents in neurodegenerative disease,9 selective fatty acid amide hydrolase inhibitors,10 inhibitors of stearoyl coenzyme A δ-9 desaturase,11 LTD4 receptor antagonists,12 orexin receptor antagonists 2,13 and histamine H2 antagonists.14
Imidazo[1,2-b]thiazoles have also attracted considerable interest in medicinal research in view of their uses as antitumor, antidiabetic, antitubercular, and anti-cardiovascular agents.15 In addition, imidazo[2,1-b]benzothiazoles have been reported recently to exhibit antibacterial,16 antifungal,17 anti-inflammatory,18 anticancer19 and analgesic activity.20 Imidazo[2,1-b][1,3,4]thiadiazoles are also of considerable interest in chemical and medicinal research because of their structural characteristics: the presence of four heteroatoms and two condensed privileged heterocyclic systems with different π-conjugation and incorporation of the imidazothiadiazole ring system as structural framework of several bioactive natural products and synthetic pharmaceuticals.21 Spirooxindoles, in particular, have emerged as attractive synthetic targets because of their prevalence in numerous clinical pharmaceuticals and natural alkaloids with their wide range of biopharmacological activities.22
The synthetic methods reported in the literature for the synthesis of the imidazo[1,2-b]thiazoles, imidazo[1,2-b]thiadiazoles and related compounds involved many limitations in terms of the multi-step synthetic procedures, use of volatile organic solvents, expensive catalysts, higher energetic reaction conditions, lower product yields and longer reaction time.23,24 Imidazo[1,2-b]thiazoles were synthesized also by isocyanide based multicomponent reaction using 2-aminobenzothiazole derivatives, quinoline-3-carbaldehyde/1H-indole-3-carbaldehyde and p-fluorophenyl isocyanide, but the reaction required 12 h for completion and provided 53–71% yields of the products.25 In view of the existing synthetic methods of imidazothiazoles, development of environmentally benign, diversity oriented, efficient and high-yielding synthetic protocol is highly desirable observing the concept of atom-economy and high convergence without isolation and purification of intermediates.
Encouraged by the promising biological activities of the heterocyclic privileged substructures and our continued research programme on the syntheses of therapeutically interesting heterocycles,26–29 we wish to report, for the first time, nanocrystalline TiO2 catalyzed one-pot three-component isocyanide-based synthesis of spiro-heterocycles incorporating privileged heterocyclic substructures, imidazothiazole/imidazothiadiazole, in aqueous ethanol in excellent yields. Our main concern is to develop a sustainable synthetic methodology involving use of recyclable and reusable nanocatalyst with mild reaction conditions.
Results and discussion
In this context, we present highly efficient and convenient one-pot synthesis of spiroheterocycles incorporating medicinally privileged heterosystems involving the reaction of 2-aminobenzothiazole/2-amino-1,3,4-thiadiazole with cycohexyl/tert-butyl isocyanides and isatines/cyclic carbonyl compounds in the presence of reusable TiO2 nanopowder as catalyst in aqueous ethanol. Initially, three-component reaction of 2-aminobenzothiazole, cycohexyl isocyanides and isatin was selected as a simple model reaction to establish the feasibility of the present synthetic strategy and to optimize the reaction conditions using different catalysts (Scheme 1).It was observed that only a trace amount of product was obtained when the reaction was carried out in solvent free and catalyst free conditions (Table 1, entry 1). The model reaction was also performed in various acid catalysts such as boric acid, p-TSA, sulfamic acid, and trifluoroacetic acid, but the desired product was obtained in moderate yield (Table 1, entries 2–5). However, when the reaction was performed in the presence of ZnS nanoparticles as catalyst in ethanol as solvent, the yield of the desired product was good (Table 1, entry 6). The reaction was also examined in the presence of commercial TiO2 and TiO2 nanoparticles as catalysts (Table 1, entries 7 and 8). The results clearly indicate that when the reaction was catalyzed with TiO2 nanoparticles in ethanol as solvent, the excellent yield of the desired product was obtained with high purity and in shorter reaction time than that obtained with the use of other catalysts. From the evaluation of screening results, it is clearly evident that TiO2 nanoparticles exhibited the largest surface areas and hence the most reactive acidic sites due to its nano-sized nature and showed superior catalytic activity over the other catalysts. The effect of catalyst loading was also examined by varying the loading amount to 10%, 15%, 20% and observed that 15 mol% of TiO2 NPs provided the maximum yield (Table 1, entry 9). The solvent effect on the catalytic efficiency of TiO2 nanoparticles was also examined employing various protic and aprotic solvents (Table 1, entries 11–15) and observed that its catalytic efficiency was maximum in aqueous ethanol (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water: v
water: v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v: 2
v: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as compared with other solvents. The effect of temperature on catalytic activity of TiO2 nanoparticles was also examined and it was observed that 90 °C was optimum temperature for maximum catalytic efficiency of TiO2 nanoparticles (Table 2).
3) as compared with other solvents. The effect of temperature on catalytic activity of TiO2 nanoparticles was also examined and it was observed that 90 °C was optimum temperature for maximum catalytic efficiency of TiO2 nanoparticles (Table 2).
| S.no. | Catalyst (mol%) | Solventc | Time (h) | Yieldd (%) |
|---|---|---|---|---|
| a Bold row indicates the optimization condition for the reaction.b 2-aminobenzothiazole (1 mmol), cycohexyl isocyanides (1 mmol) and simple isatin (1 mmol) were stirred at 90 °C till completion as indicated by TLC.c Solvents (2.0 mL).d Isolated yield after purification.e Isolated yield when commercial TiO2 catalyst was finely grounded in a mortar/pestle prior to reaction. | ||||
| 1. | Catalyst free | None | >10 | Trace |
| 2. | Boric acid (15 mol%) | Ethanol | 7 | 45 |
| 3. | Sulfamic acid (15 mol%) | Ethanol | 8 | 60 |
| 4. | p-TSA (15 mol%) | Ethanol | 7 | 60 |
| 5. | Trifluoroacteic acid (15 mol%) | Ethanol | 7 | 65 |
| 6. | ZnS NPs (15 mol%) | Ethanol | 5 | 70 |
| 7. | Commercial TiO2 (15 mol%) | Ethanol | 5 | 78, 80e |
| 8. | TiO2 NPs (10 mol%) | Ethanol | 4 | 90 |
| 9. | TiO2 NPs (15 mol%) | Ethanol | 4 | 92 |
| 10. | TiO2 NPs (20 mol%) | Ethanol | 4 | 92 |
| 11. | TiO2 NPs (15 mol%) | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (v/v: 2 water (v/v: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
3 | 94 |
| 12. | TiO2 NPs (15 mol%) | 1,4-Dioxane | 3.5 | 80 |
| 13. | TiO2 NPs (15 mol%) | Dicholoromethane | 4 | 84 |
| 14. | TiO2 NPs (15 mol%) | Tetrahydrofuran | 4 | 85 |
| 15. | TiO2 NPs (15 mol%) | Methanol | 3.5 | 89 |
| S.no. | Catalyst | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 2-aminobenzothiazole (1 mmol), cycohexyl isocyanides (1 mmol), and simple isatin (1 mmol); catalyst: TiO2 NPs (15 mol%); solvent: 2.0 mL aqueous ethanol (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water: v water: v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v: 2 v: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); at different temperatures.b Isolated yields after purification. 3); at different temperatures.b Isolated yields after purification. |
||||
| 1. | TiO2 NPs (15 mol%) | 50 °C | 4.5 | 80 |
| 2. | TiO2 NPs (15 mol%) | 60 °C | 4.15 | 80 |
| 3. | TiO2 NPs (15 mol%) | 80 °C | 3.5 | 82 |
| 4. | TiO2 NPs (15 mol%) | 90 °C | 3 | 94 |
Additionally, catalytic recyclability and reusability was also investigated on the model reaction under optimized reaction conditions. The catalyst was easily recovered by the filtration after completion of the reaction. Then it was washed with diethyl ether and then first dried under vacuum followed by drying in the oven completely. Recovered TiO2 could be used for five times without significant loss of its activity but after this, sudden decrease in yield was observed probably due to deactivation of the catalyst (Fig. 1). However, post treatment of the catalyst by calcinations at 500 °C for 1 h restored its catalytic activity and then further could be reused for three more reaction cycles (Fig. 2). The reusability of the commercial TiO2 was also observed and the results are presented in Fig. 3. Ultimately, synthesized acidic TiO2 nanoparticles preserved its activity and thermal stability and showed superior behavior over commercial TiO2 catalyst in terms of recyclability and reusability which is very important factor for large scale synthesis.
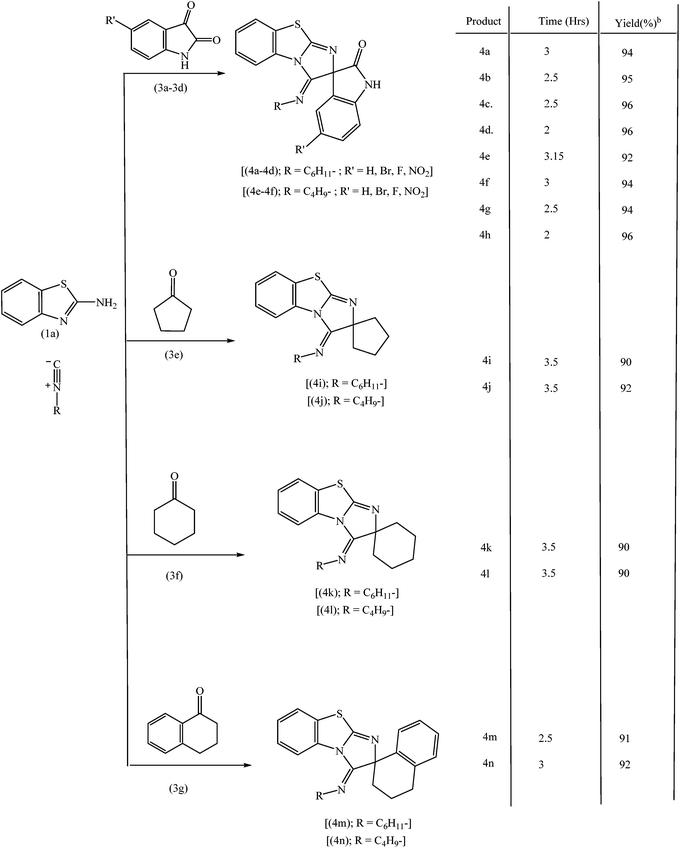
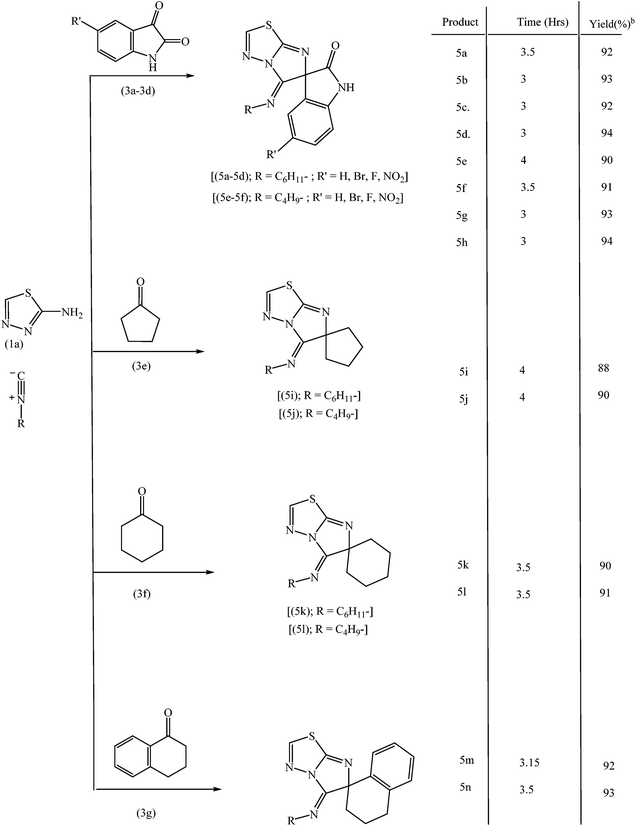
Under the optimized reaction condition, this novel isocyanide based three component reaction was extended for the synthesis of structurally diverse spiroheterocycles to explore the scope and generality of reaction protocol. To over delight, the reaction proceeds smoothly and the structurally diverse spiroheterocycles were obtained in excellent yields. The results are summarized in Tables 3 and 4.
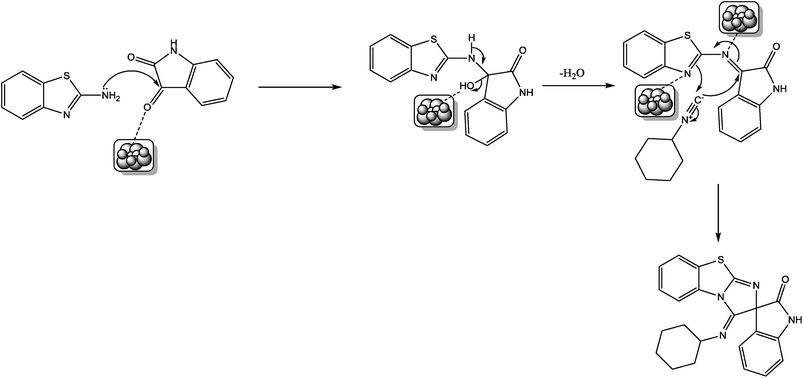
A proposed mechanism for the formation of the products is presented in Scheme 2. In the first step, nucleophilic attack of amino group of 2-aminobenzothiazole is facilitated on carbonyl carbon by coordination of TiO2 nanoparticles with carbonyl oxygen of isatin with the formation of imine intermediate. Nucleophilic attack of endocyclic nitrogen of benzothiazole moiety on carbon of cyclohexyl nitrile group and simultaneous attack of cyclohexyl nitrile carbon on imino carbon of imine intermediate provides the desired product involving cyclization.
Experimental
General procedure
The melting points of all the synthesized compounds were determined on electric melting point apparatus and are uncorrected. 2-aminobenzothiazole/2-amino-1,3,4-thiadiazole, cycohexyl/tertiary butyl isocyanides and isatines/cyclic carbonyl compounds used in the synthesis of complex heterocycles were purchased from the commercial sources and were used as such. The purity of all the synthesized compounds was checked by TLC. IR spectra were recorded on Shimadzu 8400S FTIR spectrometer. 1H NMR and 13C NMR were recorded on Bruker 300 MHz and 75 MHz NMR spectrometer, respectively. Analytical and spectral data of the synthesized heterocycles are also included.Typical procedure for synthesis of TiO2 nanoparticles
TiO2 nanoparticles were synthesized using chemical precipitation method. Titanium(III) chloride (TiCl3) was mixed with ammonium hydroxide (NH4OH) aqueous solution in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ratio respectively. Resultant solution was stirred for 48 h at room temperature. White precipitate was formed. Precipitate was separated by centrifuging the solution. In order to remove side products precipitate was washed 3–4 times with isopropyl alcohol and dried at room temperature.
3 v/v ratio respectively. Resultant solution was stirred for 48 h at room temperature. White precipitate was formed. Precipitate was separated by centrifuging the solution. In order to remove side products precipitate was washed 3–4 times with isopropyl alcohol and dried at room temperature.
Typical procedure for synthesis of spiroquinazolinones
A mixture of 2-aminobenzothiazole/2-amino-1,3,4-thiadiazole (1 mmol), cycohexyl/tertiary butyl isocyanides (1 mmol) and isatines/cyclic carbonyl compounds (1 mmol) and TiO2 nanoparticles (15 mol%) in 2.0 mL aqueous ethanol (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water: v
water: v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v: 2
v: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was stirred at 90 °C for about 2 to 4 h. After the completion of the reaction (monitored by TLC), the catalyst was recovered by filtration. The synthesized compounds were purified by column chromatography (silica gel as stationary phase, hexane–ethyl acetate (7
3) was stirred at 90 °C for about 2 to 4 h. After the completion of the reaction (monitored by TLC), the catalyst was recovered by filtration. The synthesized compounds were purified by column chromatography (silica gel as stationary phase, hexane–ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent). The catalyst was reused for the next reactions after washing it with diethyl ether.
3) as eluent). The catalyst was reused for the next reactions after washing it with diethyl ether.
The analytical and spectral data of representative compounds have been presented and the analytical and spectral data of all the synthesized compounds are included in the ESI† section of the paper.
Compounds names and spectral details
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.75–7.75 (m, 8H, ArH), 10.74 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 24.5, 25.6, 33.4, 58.6, 84.5, 116.3, 119.7, 122.2, 123.4, 124.7, 125.8, 127.1, 127.6, 128.4, 129.3, 142.3, 146.2, 158.9, 165.2, 169.6. Anal. calcd for C22H20N4OS: C 68.02, H 5.19, N 14.42%; found: C 67.94, H 5.11, N 14.36%.
C), 6.75–7.75 (m, 8H, ArH), 10.74 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 24.5, 25.6, 33.4, 58.6, 84.5, 116.3, 119.7, 122.2, 123.4, 124.7, 125.8, 127.1, 127.6, 128.4, 129.3, 142.3, 146.2, 158.9, 165.2, 169.6. Anal. calcd for C22H20N4OS: C 68.02, H 5.19, N 14.42%; found: C 67.94, H 5.11, N 14.36%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.56–7.61 (m, 4H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.5, 25.6, 27.3, 33.4, 36.1, 59.4, 69.8, 120.2, 122.3, 123.6, 125.1, 127.5, 146.3, 158.9, 165.2. Anal. calcd for C19H23N3S: C 70.12, H 7.12, N 12.91%; found: C 70.05, H 7.06, N 12.84%.
C), 6.56–7.61 (m, 4H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.5, 25.6, 27.3, 33.4, 36.1, 59.4, 69.8, 120.2, 122.3, 123.6, 125.1, 127.5, 146.3, 158.9, 165.2. Anal. calcd for C19H23N3S: C 70.12, H 7.12, N 12.91%; found: C 70.05, H 7.06, N 12.84%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.56–7.61 (m, 4H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.3, 24.5, 25.6, 25.8, 32.7, 33.5, 59.1, 64.6, 120.3, 122.3, 123.5, 125.6, 127.4, 146.2, 158.8, 164.2. Anal. calcd for C20H25N3S: C 70.76, H 7.42, N 12.38%; found: C 70.71, H 7.36, N 12.31%.
C), 6.56–7.61 (m, 4H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.3, 24.5, 25.6, 25.8, 32.7, 33.5, 59.1, 64.6, 120.3, 122.3, 123.5, 125.6, 127.4, 146.2, 158.8, 164.2. Anal. calcd for C20H25N3S: C 70.76, H 7.42, N 12.38%; found: C 70.71, H 7.36, N 12.31%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.60–7.62 (m, 8H, ArH). 13C NMR (DMSO-d6) δ (ppm): 14.6, 24.5, 25.6, 30.1, 33.4, 35.4, 59.1, 73.5, 120.3, 122.8, 123.1, 125.2, 125.9, 126.8, 127.3, 136.2, 145.7, 146.3, 158.5, 165.2. Anal. calcd for C24H25N3S: C 74.38, H 6.50, N 10.84%; found: C, H, N%.
C), 6.60–7.62 (m, 8H, ArH). 13C NMR (DMSO-d6) δ (ppm): 14.6, 24.5, 25.6, 30.1, 33.4, 35.4, 59.1, 73.5, 120.3, 122.8, 123.1, 125.2, 125.9, 126.8, 127.3, 136.2, 145.7, 146.3, 158.5, 165.2. Anal. calcd for C24H25N3S: C 74.38, H 6.50, N 10.84%; found: C, H, N%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.10–8.05 (m, 5H, ArH), 10.78 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 24.7, 25.6, 33.5, 58.3, 84.1, 116.3, 125.7, 127.1, 127.9, 129.3, 142.3, 148.7, 158.5, 164.8, 169.6. Anal. calcd for C17H17N5OS: C 60.16, H 5.05, N 20.63%; found: C 60.08, H 4.98, N 20.55%.
C), 7.10–8.05 (m, 5H, ArH), 10.78 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 24.7, 25.6, 33.5, 58.3, 84.1, 116.3, 125.7, 127.1, 127.9, 129.3, 142.3, 148.7, 158.5, 164.8, 169.6. Anal. calcd for C17H17N5OS: C 60.16, H 5.05, N 20.63%; found: C 60.08, H 4.98, N 20.55%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 8.02 (s, 1H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.7, 25.5, 27.3, 33.5, 36.3, 58.9, 67.7, 148.7, 158.6, 164.8. Anal. calcd for C14H20N4S: C 60.84, H 7.29, N 20.27%; found: C 60.72, H 7.21, N 20.17%.
C), 8.02 (s, 1H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.7, 25.5, 27.3, 33.5, 36.3, 58.9, 67.7, 148.7, 158.6, 164.8. Anal. calcd for C14H20N4S: C 60.84, H 7.29, N 20.27%; found: C 60.72, H 7.21, N 20.17%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 8.04 (s, 1H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.1, 24.7, 25.4, 25.8, 31.3, 33.6, 58.7, 63.8, 148.6, 158.5, 165.3. Anal. calcd for C15H22N4S: C 62.03, H 7.64, N 19.29%; found: C 61.91, H 7.53, N 19.23%.
C), 8.04 (s, 1H, ArH). 13C NMR (DMSO-d6) δ (ppm): 24.1, 24.7, 25.4, 25.8, 31.3, 33.6, 58.7, 63.8, 148.6, 158.5, 165.3. Anal. calcd for C15H22N4S: C 62.03, H 7.64, N 19.29%; found: C 61.91, H 7.53, N 19.23%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.80–8.03 (m. 5H, ArH). 13C NMR (DMSO-d6) δ (ppm): 14.6, 24.6, 25.7, 30.4, 33.3, 35.1, 59.0, 73.8, 125.2, 127.3, 136.4, 145.3, 148.5, 158.7, 165.3. Anal. calcd for C19H22N4S: C 67.42, H 6.55, N 16.55%; found: C 67.34, H 6.48, N 16.48%.
C), 6.80–8.03 (m. 5H, ArH). 13C NMR (DMSO-d6) δ (ppm): 14.6, 24.6, 25.7, 30.4, 33.3, 35.1, 59.0, 73.8, 125.2, 127.3, 136.4, 145.3, 148.5, 158.7, 165.3. Anal. calcd for C19H22N4S: C 67.42, H 6.55, N 16.55%; found: C 67.34, H 6.48, N 16.48%.Conclusion
In conclusion, we have presented an efficient and environmentally benign isocyanide-based domino protocol for the synthesis of structurally diverse spiroheterocycles spiroannulated with imidazothiazole and imidazothiadiazole involving three-component reaction of 2-aminobenzothiazole/2-amino-1,3,4-thiadiazole, cyclohexyl/tert-butyl isocyanides and isatines/cyclic carbonyl compounds catalyzed by recyclable and reusable nanocrystalline TiO2. The present synthetic protocol is probably the first report on isocyanide-based nanocatalyzed multicomponent synthesis of spiroheterocycles and offers several advantages such as operational simplicity with easy workup, shorter reaction times, excellent yields with superior atom economy and environmentally benign reaction conditions with the use of recyclable, reusable, non-toxic catalyst and aqueous ethanol as green solvent. Moreover, easy separation of the catalyst by simple filtration after completion of the reaction, and reusability of the catalyst for even 8 repeated reaction cycles (after simple calcinations treatment) make this protocol interesting for large scale synthesis.Acknowledgements
We gratefully acknowledge UGC New Delhi for the award of Research Fellowships (Y.K.T.). Head, Department of Chemistry is acknowledged for providing Lab and instrumental facilities in the department. Director, SAIF, Panjab University, Chandigarh and Therachem, Jaipur are also acknowledged for elemental analysis and spectra of the synthesized compounds.References
- (a) L. F. Tietze and A. Modi, Med. Res. Rev., 2000, 20, 304 CrossRef CAS; (b) M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef PubMed; (c) Domino Reactions in Organic Synthesis, ed. L. F. Tietze, G. Brasche and K. M. Gericke, Wiley-VCH, Weinheim, Germany, 2006 Search PubMed; (d) G. Morel, E. Marchand and A. Foucaud, J. Org. Chem., 1985, 50, 771 CrossRef CAS; (e) G. Morel, E. Marchand, A. Foucaud and L. Toupet, J. Org. Chem., 1989, 54, 1185–1191 CrossRef CAS; (f) K. Burger, U. Wassmuth and S. Penninger, J. Fluorine Chem., 1982, 20, 813 CrossRef CAS.
- (a) X. H. Chen, X. Y. Xu, H. Liu, L. F. Cun and L. Z. Gong, J. Am. Chem. Soc., 2006, 128, 14802 CrossRef CAS PubMed; (b) D. A. Horton, T. G. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (c) J. P. Kennedy, L. Williams, T. M. Bridges, R. N. Daniels, D. Weaver and C. W. Lindsley, J. Comb. Chem., 2008, 10, 345 CrossRef CAS PubMed.
- (a) P. Hervés, M. P. Lorenzo, L. M. Liz-Marzán, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577 RSC; (b) B. S. Takale, M. Bao and Y. Yamamoto, Org. Biomol. Chem., 2014, 12, 2005 RSC.
- S. Santra, A. K. Bagdi, A. Majee and A. Hajra, RSC Adv., 2013, 3, 24931 RSC.
- (a) S. Rana, M. Brown, A. Dutta, A. Bhaumik and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 1371 CrossRef CAS PubMed; (b) S. Abdolmohammadi, Chin. Chem. Lett., 2013, 24, 318 CrossRef CAS PubMed; (c) A. Mondal, S. Rana and C. Mukhopadhyay, Tetrahedron Lett., 2014, 55, 3498 CrossRef CAS PubMed.
- (a) M. M. Ba-Abbad, A. A. H. Kadhum, A. B. Mohamad, M. S. Takriff and K. Sopian, Int. J. Electrochem. Sci., 2012, 7, 4871 CAS; (b) C. H. Kuo, A. S. Poyraz, L. Jin, Y. Meng, L. Pahalagedara, S. Y. Chen, D. A. Kriz, C. Guild, A. Gudza and S. L. Suib, Green Chem., 2014, 16, 785 RSC; (c) A. Ayati, A. Ahmadpour, F. F. Bamoharram, B. Tanhaei, M. Mänttäri and M. Sillanp, Chemosphere, 2014, 107, 163 CrossRef CAS PubMed.
- (a) J. Ma, D. Chen, K. Lu, L. Wang, X. Han, Y. Zhao and P. Gong, Eur. J. Med. Chem., 2014, 86, 257 CrossRef CAS PubMed; (b) N. Karalı, O. Guzel, N. Ozsoy, S. Ozbey and A. Salman, Eur. J. Med. Chem., 2010, 45, 1068 CrossRef PubMed; (c) A. Kamal, Md. Ashraf, M. V. P. S. V. Vardhan, S. Faazil and V. L. Nayak, Bioorg. Med. Chem. Lett., 2014, 24, 147 CrossRef CAS PubMed.
- (a) R. S. Keri, M. R. Patil, S. A. Patil and S. Budagumpi, Eur. J. Med. Chem., 2015, 89, 207 CrossRef CAS PubMed; (b) M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary and M. M. El-Kerdawy, Eur. J. Med. Chem., 2014, 85, 576 CrossRef CAS PubMed; (c) M. K. Singh, R. Tilak, G. Nath, S. K. Awasthi and A. Agarwal, Eur. J. Med. Chem., 2013, 63, 635 CrossRef CAS PubMed; (d) S. Ke, Y. Wei, Z. Yang, K. Wang, Y. Liang and L. Shi, Bioorg. Med. Chem. Lett., 2013, 23, 5131 CrossRef CAS PubMed; (e) S. Banerjee, S. Payra, A. Saha and G. Sereda, Tetrahedron Lett., 2014, 55, 5515 CrossRef CAS PubMed.
- G. Henriksen, A. I. Hauser, A. D. Westwell, B. H. Yousefi, M. Schwaiger, A. Drzega and H. J. Wester, J. Med. Chem., 2007, 50, 1087 CrossRef CAS PubMed.
- X. Wang, K. Sarris, K. Kage, D. Zhang, S. P. Brown, T. Kolasa, C. Surowy, O. F. ElKouhen, S. W. Muchmore, J. D. Brioni and A. O. Stewart, J. Med. Chem., 2009, 52, 170 CrossRef CAS PubMed.
- C. Black, D. Deschenes, M. Gagnon, N. Lachance, Y. Leblanc, S. Leger, C. S. Li and R. M. Oballa, PCT Int Appl, WO 2006122200 A1 20061116, 2006.
- C. K. Lau, C. Dufresne, Y. Gareau, R. Zamboni, M. Labelle, R. N. Young, K. M. Metters, C. Rochette, N. Sawyer, D. M. L. Slipetz, C. T. Jones, M. McAuliffe, C. McFarlane and A. W. Ford-Hutchinson, Bioorg. Med. Chem., 1995, 5, 1615 CrossRef CAS.
- J. M. Bergman, P. J. Coleman, C. Cox, G. D. C. Hartman Lindsley, S. P. Mercer, A. J. Roecker and D. B. Whitman, PCT Int. Appl, WO 2006127550, 2006.
- J. Apelt, S. Grassmann, X. Ligneau, H. H. Pertz, C. R. Ganellin, J. M. Arrang, J. C. Schwartz, W. Schunack and H. Stark, Pharmazie, 2005, 60, 97 CAS.
- (a) J. H. Park, M. I. El-Gamal, Y. S. Lee and C. H. Oh, Eur. J. Med. Chem., 2011, 46, 5769 CrossRef CAS PubMed; (b) C. B. Vu, J. E. Bemis, J. S. Disch, P. Y. Ng, J. J. Nunes, J. C. Milne, D. P. Carney, A. V. Lynch, J. J. Smith, S. Lavu, P. D. Lambert, D. J. Gagne, M. R. Jirousek, S. Schenk, J. M. Olefsky and R. B. Perni, J. Med. Chem., 2009, 52, 1275 CrossRef CAS PubMed; (c) G. C. Moraski, L. D. Markley, M. Chang, S. Cho, S. G. Franzblau, C. H. Hwang, H. Boshoff and M. Miller, Bioorg. Med. Chem., 2012, 20, 2214 CrossRef CAS PubMed; (d) A. Locatelli, S. Cosconati, M. Micucci, A. Leoni, L. Marinelli, A. Bedini, P. Loan, S. M. Spampinato, E. Novellino, A. Chiarini and R. Budriesi, J. Med. Chem., 2013, 56, 3866 CrossRef CAS PubMed.
- M. Palkar, M. Noolvi, R. Sankangoud, V. Maddi, A. Gadad and L. V. Nargund, Arch. Pharm., 2010, 343, 353 CrossRef CAS PubMed.
- J. K. Malik, H. Soni and A. K. Singhai, J. Pharm. Res., 2013, 7, 39 CrossRef CAS PubMed.
- A. N. El-Shorbagi, S. Sakai, M. A. el-Gendy, N. Omar and H. H. Farag, Chem. Pharm. Bull., 1989, 37, 2971 CrossRef CAS.
- R. M. Kumbhare, K. V. Kumar, M. J. Ramaiah, T. Dadmal, S. N. C. V. L. Pushpavalli, D. Mukhopadhyay, B. Divya, T. A. Devi, U. Kosurkar and M. Pal-Bhadra, Eur. J. Med. Chem., 2011, 46, 4258 CrossRef CAS PubMed.
- F. Palagiano, L. Arenare, P. De Caprariis, G. Grandolini, V. Ambrogi, L. Perioli, W. Filippelli, G. Falcone and F. Rossi, Farmaco, 1996, 51, 483 CAS.
- (a) B. A. Bhongade, S. Talath, R. A. Gadad and A. K. Gadad, J. Saudi Chem. Soc., 2013 DOI:10.1016/j.jscs.2013.01.010 , in press; (b) I. A. M. Khazi, A. K. Gadad, R. S. Lamani and B. A. Bhongade, Tetrahedron, 2011, 67, 3289 CrossRef CAS PubMed.
- (a) M. M. M. Santos, Tetrahedron, 2014, 70, 9735 CrossRef CAS PubMed; (b) Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673 CrossRef CAS PubMed; (c) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed.
- (a) J. Gao, J. Zhu, L. Chen, Y. Shao, J. Zhu, Y. Huang, X. Wang and Xin Lv, Tetrahedron Lett., 2014, 55, 3367 CrossRef CAS PubMed; (b) M. S. Christodoulou, F. Colombo, D. Passarella, G. Ieronimo, V. Zuco, M. De Cesare and F. Zunino, Bioorg. Med. Chem., 2011, 19, 1649 CrossRef CAS PubMed.
- H. M. Patel, M. N. Noolvi, N. S. Sethi, A. K. Gadad and S. S. Cameotra, Arabian J. Chem., 2013 DOI:10.1016/j.arabjc.2013.01.001 , in press.
- T. H. Al-Tel, R. A. Al-Qawasmeh and R. Zaarour, Eur. J. Med. Chem., 2011, 46, 1874 CrossRef CAS PubMed.
- (a) A. Rajawat, S. Khandelwal and M. Kumar, RSC Adv., 2014, 4, 5105 RSC; (b) S. Khandelwal, A. Rajawat and M. Kumar, Curr. Bioact. Compd., 2013, 9, 203 CrossRef CAS; (c) A. K. Arya and M. Kumar, Mol. Diversity, 2011, 15, 781 CrossRef CAS PubMed.
- (a) A. K. Arya, S. K. Gupta and M. Kumar, Tetrahedron Lett., 2012, 53, 6035 CrossRef CAS PubMed; (b) M. Kumar, K. Sharma and A. K. Arya, Tetrahedron Lett., 2012, 53, 4604 CrossRef CAS PubMed.
- (a) A. K. Arya and M. Kumar, Green Chem., 2011, 13, 1332 RSC; (b) M. Kumar, K. Sharma, D. K. Sharma and A. K. Arya, Tetrahedron Lett., 2013, 54, 878 CrossRef CAS PubMed; (c) S. Khandelwal, A. Rajawat and M. Kumar, Curr. Organocatal., 2014, 1, 51 CrossRef CAS.
- (a) S. K. Gupta, A. K. Arya, S. Khandelwal and M. Kumar, Curr. Org. Chem., 2014, 18, 2555 CrossRef CAS; (b) S. Khandelwal, A. Rajawat, Y. K. Tailor and M. Kumar, Comb. Chem. High Throughput Screening, 2014, 17, 763 CrossRef CAS; (c) S. Khandelwal, A. Rajawat, Y. K. Tailor and M. Kumar, Curr. Organocatal., 2014, 2, 37–43 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04863j |
| This journal is © The Royal Society of Chemistry 2015 |