Anti-degradation gelatin films crosslinked by active ester based on cellulose†
Chen Zhuang,
Furong Tao and
Yuezhi Cui*
Shandong Provincial Key Laboratory of Fine Chemicals, Qilu University of Technology, Jinan 250353, P. R. China. E-mail: yuezhicui@163.com; Fax: +86 531 89631760; Tel: +86 531 89631208
First published on 2nd June 2015
Abstract
Functionalization of microcrystalline cellulose (MCC) with EDTA dianhydride (EDTAD) was first achieved using an esterification reaction. N-Hydroxysuccinimide-activated MCC-EDTAD ester (MEN), a novel macromolecule crosslinker based on MCC, was synthesized for the modification of gelatin films. The reaction between gelatin and MEN was verified by the residual free amino test, FTIR and XRD spectra. The introduction of MEN into gelatin decreased the film degradation ratio and increased its thermal stability, flexibility, hydrophobicity, light barrier performance and water uptake ability. Additionally, SEM images proved the successful surface grafting reaction and degradation phenomenon. This unique gelatin film material with advanced properties broke the limitation of the blending method for modification of gelatin with macromolecules and broadened its application as a novel sustained-release material.
1. Introduction
Gelatin is a peptide-based polymeric material, obtained via the hydrolytic treatment of collagen under acidic or alkaline conditions. The triple helix structure of collagen partially separates and ruptures, and a non-uniform mixture of polypeptides with different amino acids are formed.1 Because of its good biological properties and low toxicity, gelatin is widely used for different kinds of materials, such as sponges, films, microballoons, scaffolds, nanoparticles, bandages, etc.2–6 However, the relatively weak thermal stability, poor mechanical properties and easily-degradable quality limit the potential application of gelatin as a practical material.7 Microcrystalline cellulose (MCC), a linear polysaccharide comprising β-glucoside units, is usually blended with gelatin to overcome the obstacles of the biopolymer matrix.8–10 Its excellent properties, such as renewable origin, biodegradability of its components, and environment-friendly and non-toxic character, further broaden its usages.10–13 Ethylenediamine tetraacetic dianhydride (EDTAD) is commonly used as a chelating reagent.14 Its biodegradable behavior and special molecular structure, which consists of two anhydride groups that can react with hydroxyl or amine groups, ensure its function in the modification of biomaterials.15,16In recent years, a great number of researchers worldwide have been devoted to the modification of gelatin with various crude macromolecules, such as cellulose, chitosan, starch, montmorillonite, polyvinyl alcohol, zeolite, etc.17–22 Jridi et al.23 investigated the physical, structural, antioxidant and antimicrobial properties of gelatin/chitosan composite films and chose the best proportion of the two components to be applied as a food packaging material; Li et al.24 prepared active gelatin-based films incorporating five kinds of natural antioxidants and compared the effect of these extracts on the antioxidant, physical and mechanical properties of the films; Alves et al.25 studied the effect of three components (gelatin, cellulose, starch) on the biodegradation, water vapor permeability and mechanical properties of the starch/cellulose/gelatin nanocrystal films using orthogonal experiments; Andrade et al.26 reported a new edible coating material containing gelatin and cellulose nanofibers, and evaluated the wettability of the coating film on banana and eggplant epicarps. Unfortunately, the existing modification of gelatin-based composite films with natural polymers, especially cellulose, is mostly achieved using a blending method, in which the hydrogen bonding or electrostatic interactions are used to explain the reaction mechanism of the polymer matrix. No exact chemical reaction occurs between the gelatin and the original cellulose. Therefore, proper chemical modification of cellulose is needed to make the crosslinking reaction with gelatin possible. Cheng et al.27 oxidized cellulose by periodate oxidation to obtain 2,3-dialdehyde cellulose (DARC), which then reacted with collagen via a Schiff base reaction between –NH2 in the collagen and –CHO in the DARC backbone to obtain DARC/Col composite films; Li et al.28 employed the same oxidation process to oxidize carboxymethyl cellulose, and the product, with two aldehyde groups, reacted with gelatin to prepare an edible film material.
Recently, a novel crosslinker, N-hydroxysuccinimide (NHS) active ester, which is synthesized via the reaction between carboxylic acid and NHS in the presence of a carbodiimide,29 has attracted a substantial amount of attention, mainly due to its cytocompatibility, biocompatibility and availability.30,31 Furthermore, Gil’s group32 has concentrated on modifying sugarcane bagasse, which is a raw material of cellulose, with EDTAD to gain the ester group for its use as an absorbent material. In light of this research, the hydroxyl and/or carboxyl functional groups in these three biological polymers (gelatin, cellulose and EDTAD) further guaranteed the chemical reaction to produce materials with new properties.33
In this paper, microcrystalline cellulose was modified with EDTAD to obtain a new type of cellulose ester, MCC-EDTAD (ME). Then, a novel macromolecule crosslinker, N-hydroxysuccinimide activated MCC-EDTAD ester (MCC-EDTAD-NHS, MEN), was synthesized in the presence of 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (EDC) to react with gelatin (Scheme 1), and a biological polymer film with new qualities was found. FTIR, XRD, TGA-DSC, mechanical properties, contact angles and residual amino group testing were applied in our present study. Additionally, in vitro degradation studies, light barrier properties and water uptake measurements of the crosslinked gelatin films were investigated. On the basis of these results, the comparison of the thermal stability and light barrier properties between MEN-modified gelatin films (Gel–MEN) and cellulose blending films (Gel/MCC) were explored.
 | ||
| Scheme 1 The formation process of crosslinked gelatin with MEN. (1) The synthetic route of EDTA anhydride (EDTAD); (2) the preparation path of gelatin modified with MEN. | ||
2. Experimental
2.1 Materials
Gelatin (type A, obtained from pigskin, with an approximate molecular weight of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and isoelectric point at pH = 8, determined using fluorescence measurements) was obtained from Sinopharm Chemical Reagent Co., Ltd. MCC (extra pure, average particle size 90 μm), NHS (AR, 98%) and EDC (AR, 99%) were purchased from Energy Chemical Technology Co., Ltd (Shanghai). Glycerol (AR, 99%), DMF (AR, 99.5%), EDTA disodium salt (AR, 99%), acetic anhydride (AR, 98.5%) and other agents were obtained from Tianjin Fu Yu Fine Chemical Co., Ltd. All chemicals and reagents were used as received without further purification.
000 and isoelectric point at pH = 8, determined using fluorescence measurements) was obtained from Sinopharm Chemical Reagent Co., Ltd. MCC (extra pure, average particle size 90 μm), NHS (AR, 98%) and EDC (AR, 99%) were purchased from Energy Chemical Technology Co., Ltd (Shanghai). Glycerol (AR, 99%), DMF (AR, 99.5%), EDTA disodium salt (AR, 99%), acetic anhydride (AR, 98.5%) and other agents were obtained from Tianjin Fu Yu Fine Chemical Co., Ltd. All chemicals and reagents were used as received without further purification.
2.2 Preparation of MEN
For the preparation of EDTA dianhydride, 18 g EDTA was suspended in 50 ml pyridine and 25 ml acetic anhydride was added. Then, the mixture was heated under reflux and kept stirring at 65 °C for 24 h. After reaction, the solid obtained was vacuum filtered, rinsed with diethyl ether and dried under vacuum at 50 °C. The prepared EDTAD was characterized using 1H-NMR spectroscopy (Bruker Advance 400 spectrometer) and FTIR spectroscopy (Nicolet NEXUS 470 FT-IR spectrometer).
 | (1) |
2.3 Modification process and film formation
A gelatin solution (3%, w/v) was prepared by dissolving gelatin powder in distilled water and was then heated at 45 °C for 2 h under continuous stirring. Glycerol was added as a plasticizer at a certain concentration (15% of dry gelatin weight). The dosage of MEN was determined by the mass ratio with gelatin, i.e., mMEN/mgelatin = 0%, 5%, 10%, 15%, 20%, 25%, 30%. Therefore, the corresponding modified gelatin samples were named as Gel, Gel–5%MEN, Gel–10%MEN, Gel–15%MEN, Gel–20%MEN, Gel–25%MEN and Gel–30%MEN, respectively. The appropriate weight of MEN powder was dissolved in distilled water under stirring for 12 h at room temperature to produce a liquid suspension. Then, the solution was added dropwise to the gelatin liquid, and acetic acid (3% of water volume) was added dropwise into the whole system to promote the start of the interfacial reaction. These mixtures were gently stirred for 12 h at 45 °C.To cast the films, 30 g of each gelatin reaction solution was transferred into a teflon dish and placed at room temperature for 2 h, then put in an oven at 40 °C until the films dried. The dried films were peeled off and stored in a desiccator with relative humidity ≤20%. In addition, one part of gelatin reaction solution was freeze dried at −55 °C, 70 Pa with a vacuum freeze drier (FD-1A-50, Beijing, China) and the lyophilized powder was characterized using FTIR spectroscopy (Nicolet NEXUS 470 FT-IR spectrometer).
2.4 XRD analysis
XRD analysis of the samples was performed on an X-ray diffractometer (D8-ADVANCER, Bruker AXE, Germany) with a thin film attachment using Cu-Kα radiation (λ = 0.1541 nm) at a current of 40 mA and an accelerating voltage of 40 kV. The patterns were recorded from 10° to 60°.2.5 Determination of residual amino groups in gelatin
The residual –NH2 groups in the modified gelatin solution were determined using the improved Van Slyke method at 45 °C.37,38 Sample solutions were mixed with acetic acid and sodium nitrite and stirred for 45 min. The residual primary amine (mol g−1) was calculated according to the volume of N2. All samples were tested in triplicate.2.6 In vitro degradation studies
The degradation study of gelatin films was carried out in vitro by incubating in phosphate buffer (pH 7.40) at 37 °C for different intervals (1, 3, 5, 7, 9, 12 and 24 h), which was developed from the method of Haroun.39 The gelatin films were dried at 60 °C to a constant weight prior to use and marked as m0. After different degradation times, the samples were washed with distilled water after filtering under vacuum and dried at 60 °C to a constant weight (mt). The degradability performance was examined from the weight remaining using eqn (2).
 | (2) |
2.7 Scanning electron microscopy (SEM) of gelatin films
The microstructures of the prepared films were investigated using a Quanta 200 environmental scanning electron microscope (FEI Company, Holland). Before observation, the film surfaces were coated with Au using a SEM coating device. More than ten micrographs were taken from different zones of each surface film.2.8 Thermogravimetric analysis
The thermal stability of the gelatin films was determined using thermogravimetric analysis and differential thermal scanning calorimetry synchronous apparatus (TGA-DSC, Q600SDT, TA, USA). The gelatin film samples (approximately 2.5 mg) were weighed accurately into aluminium pans and sealed. The endothermal curve of the crushed film was recorded from 20 °C to 500 °C at a scanning rate of 10 °C min−1 under nitrogen atmosphere. Additionally, the thermal stability of the Gel/MCC blend film was also studied and compared with the Gel–MEN films.2.9 Mechanical testing
Prior to investigating the mechanical properties, the films were conditioned for 48 h at 20 °C and 50 ± 5% RH. Tensile strength (Ts), elasticity modulus (Em) and elongation at break (Eab) were determined as described by Benjakul40 with a slight modification, using a Microcomputer Controlled Electronic Tensile Testing Machine (WDL-005, Jinan, China) equipped with a tensile load cell of 300 N. Samples with initial grip length of 25 mm were used for testing and the cross-head speed was set at 10 mm min−1. The thickness of each film was measured using a Vernier Caliper (0.02 mm/150 mm, Shanghai, China).2.10 Contact angle measurement
The water contact angles (CAs) of all films were measured using the Sessile drop method with a DSA100 contact angle measuring system from Krüss. The gelatin reaction solution was coated on the surface of a glass sheet to obtain films with thickness of about 0.1 mm, and then stored in a desiccator with relative humidity ≤20%.2.11 Light barrier properties and transparency
The ultraviolet and visible light barrier properties of the films (1 cm × 2 cm) were measured using an ultraviolet-visible spectrophotometer (UV-7504C, Shanghai, China) at selected wavelengths from 200 to 800 nm following Fang’s method.41 The transparency values of the films were calculated using eqn (3), where T is the transmission (%) at each wavelength and x is the film thickness (mm). According to the equation, high transparency values indicate good light barrier performance.
Transparency value = −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T/x T/x
| (3) |
2.12 Water uptake measurement
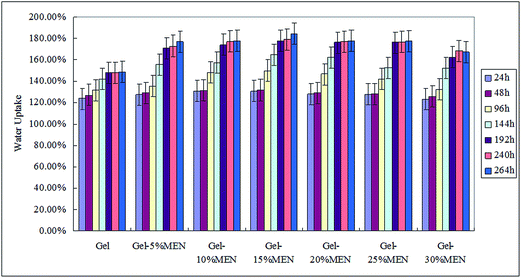
The water uptake of the films was determined similarly to Kavoosi42 and Tang,43 with a little development. Rectangular specimens sized 15 mm × 10 mm with a thickness of 0.1 mm were prepared. The samples were conditioned at 20 °C in a desiccator containing silica gel (RH 20% ± 5%) for three days, to constant weight (Wi). Then, the film samples were transferred into desiccators at 100% relative humidity (super-saturated salt solution of CuSO4·5H2O) at 20 °C for eleven days to absorb water until the weight reached equilibrium. The weights of the samples at the adsorption time t were noted as Wt. The amount of water adsorbed at different intervals and at equilibrium were calculated using eqn (4). All tests are the means of at least three measurements.
 | (4) |
3. Results and discussion
3.1 Characterization of MEN
| Materials | C (%) | H (%) | N (%) | Weight gain (%) | Ti (oC) | Tm (oC) | Tg (oC) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| MCC | 42.21 | 6.40 | 0.11 | — | 309.10 | 364.08 | 340.68 | 15.28 |
| ME | 42.29 | 6.45 | 1.92 | 72.50 | 271.45 | 331.50 | 342.70 | 33.01 |
| MEN | 42.97 | 6.65 | 2.48 | 30.80 | 222.54 | 377.31 | 364.09 | 10.45 |
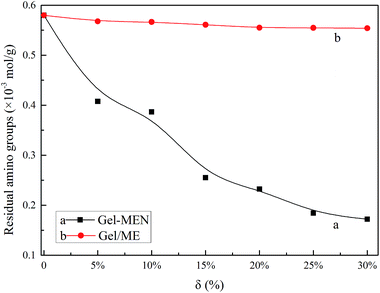
The initial decomposition temperature at 5% weight loss (Ti), the maximum weight loss temperature (Tm), the glass transition temperature (Tg) and the char residue at 500 °C of MCC, ME and MEN are recorded in Table 1 (Fig. S4†). The Ti values of MCC and ME were 309.10 °C and 271.45 °C, respectively, while that of MEN was 222.54 °C, which suggested a reduction in thermal stability. This can be related to the reactivity of the three materials with –NH2 in gelatin, which was in accord with the results of the residual amino groups, below. To summarize, the difference of each item further verified the introduction of EDTA and NHS into MCC, which agreed with the FTIR and elemental analysis.
3.2 Confirmation of MEN crosslinking with gelatin
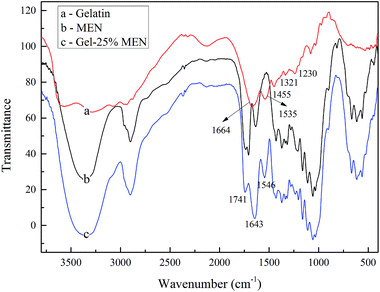
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration appearing at 1664 cm−1 demonstrated the amide I band, while the amide band II indicating the N–H bending vibration was observed at 1535 cm−1. In addition, aliphatic C–H bending vibrations were observed at 1450 cm−1 and bands at 1331 and 1230 cm−1 showed the C–N bond stretching vibrations. Gel–MEN showed all the characteristic peaks of gelatin and MEN, such as those at 1643 and 1546 cm−1, which indicated the successful reaction between gelatin and crosslinker MEN, along with a representative peak at 1741 cm−1, which clearly indicated the amidation reaction between –NH2 in gelatin and the active ester base in MEN.
O stretching vibration appearing at 1664 cm−1 demonstrated the amide I band, while the amide band II indicating the N–H bending vibration was observed at 1535 cm−1. In addition, aliphatic C–H bending vibrations were observed at 1450 cm−1 and bands at 1331 and 1230 cm−1 showed the C–N bond stretching vibrations. Gel–MEN showed all the characteristic peaks of gelatin and MEN, such as those at 1643 and 1546 cm−1, which indicated the successful reaction between gelatin and crosslinker MEN, along with a representative peak at 1741 cm−1, which clearly indicated the amidation reaction between –NH2 in gelatin and the active ester base in MEN.
3.3 Performance of Gel–MEN films
 | ||
| Fig. 5 Film surface morphology of Gel (a1, a2), Gel–25%MEN (b1, b2) and Gel–25%MEN after 1 h degradation (c1, c2). | ||
In addition, the SEM images provided very good evidence in favor of the in vitro degradation of the test sample (Gel–25%MEN). It can be seen from Fig. 5(b) and (c) that the film surface was almost planar and even, though combined with some sags and crests, before the degradation started. After one hour of degradation, a porous structure with irregularities and apertures can be observed on the surface of the composite film, which confirmed that the internal structure of the Gel–MEN polymeric film had started to degrade in the liquid medium. It can be assumed that the degradation of the films was gradually penetrating deeper from the surface.48
 | ||
| Fig. 6 TGA and DTG curves of modified gelatin films with different dosages of crosslinker (6-1 and 6-2) and comparison curves between Gel–25%MEN and Gel/25%MCC blend films (6-3 and 6-4). | ||
The thermal properties of Gel–25%MEN and Gel/25%MCC in the presence of glycerol were compared in Fig. 6-3 and 6-4, in which curve b consisted of four decomposition stages. The four peaks at 104.42, 192.23, 250.72 and 359.12 °C were resolved into four different components of water, gelatin, glycerol and MCC, respectively. The obvious peak at 192.23 °C that almost disappeared in curve a indicated the severe phase separation in the Gel/MCC system. However, compared with the typical Tm (309.10 °C) of MCC,49 the increased decomposition temperature of 359.12 °C in curve b may caused by the hydrogen bonds formed between gelatin and MCC, which increased the thermal stability of the Gel/MCC films.
| Films | Thickness (mm) | Tensile strength (MPa) | Elongation at break (%) | Elasticity modulus (MPa) |
|---|---|---|---|---|
| Gel | 0.10 | 24.17 | 1.84 | 1736.11 |
| Gel–5%MEN | 0.18 | 15.28 | 7.64 | 435.73 |
| Gel–10%MEN | 0.20 | 16.17 | 11.52 | 468.75 |
| Gel–15%MEN | 0.20 | 18.25 | 12.44 | 595.24 |
| Gel–20%MEN | 0.28 | 18.10 | 28.64 | 525.21 |
| Gel–25%MEN | 0.26 | 13.97 | 31.96 | 448.72 |
| Gel–30%MEN | 0.20 | 14.08 | 83.08 | 476.19 |
| Films | Wavelength (nm) | Transparency value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 450 | 500 | 550 | 600 | 700 | 800 | 280 | 600 | |
| Gel | 2.2 | 1.5 | 43.2 | 55.0 | 59.7 | 62.6 | 64.4 | 66.5 | 67.6 | 68.5 | 10.13 | 0.98 |
| Gel–MEN5% | 1.5 | 5.8 | 29.8 | 34.4 | 35.4 | 36.4 | 37.0 | 37.8 | 38.4 | 39.0 | 7.73 | 2.64 |
| Gel–MEN10% | 0.6 | 2.6 | 12.4 | 14.4 | 15.1 | 15.5 | 15.8 | 16.2 | 16.4 | 16.4 | 11.32 | 5.65 |
| Gel–MEN15% | 0.2 | 0.2 | 4.4 | 5.8 | 6.3 | 6.6 | 6.7 | 7.1 | 7.1 | 7.2 | 13.49 | 5.74 |
| Gel–MEN20% | 0.1 | 0.2 | 2.2 | 3.0 | 3.5 | 3.6 | 3.7 | 3.7 | 3.7 | 3.7 | 13.49 | 7.16 |
| Gel–MEN25% | 0 | 0 | 0.6 | 1.0 | 1.3 | 1.4 | 1.5 | 1.6 | 1.6 | 1.6 | — | 8.98 |
| Gel–MEN30% | 0 | 0 | 0.4 | 0.6 | 0.9 | 1.0 | 1.1 | 1.1 | 1.1 | 1.1 | — | 9.79 |
| Gel/MCC15% | 1.9 | 9.3 | 34.6 | 39.6 | 40.9 | 42.3 | 43.3 | 45.1 | 46.5 | 47.7 | 10.32 | 3.46 |
| Gel/MCC25% | 1.4 | 5.3 | 26.6 | 31.7 | 33.0 | 34.4 | 35.5 | 36.9 | 38.0 | 39.3 | 12.78 | 4.32 |
Table 3 also displays the light barrier properties of Gel/15%MCC and Gel/25%MCC blend films, as compared with the corresponding mass ratio of Gel–MEN films. The Gel/MCC blend films exhibited better transparency but worse light resistance performance. This fact may be an indication that MCC nanoparticles were homogeneously distributed in the matrix because they are white, and light incident on the film surface was reflected in a larger quantity due to the white particles.25 The light barrier properties of the films are relatively important when used as sustained-release materials for food packaging or food coating. The polymer matrix in this work matches these needs.
4. Conclusion
In summary, the structure and conformation of gelatin were modified by the macromolecule crosslinker MEN. The FTIR spectra, elemental analysis and TGA values verified the structure of MEN. The reaction between –NH2 in gelatin and the active ester in MEN was confirmed by the residual primary amino groups test, and the FTIR and XRD spectra, which broke the limitations of the blending method for modifying gelatin with macromolecules. The dose-dependent effect of the crosslinker was investigated through degradation in vitro, in which the weight remaining decreased with the increase in MEN dosage. The SEM images further proved the successful surface grafting reaction and the degradation phenomenon in PBS medium. The decomposition temperature obtained from TGA curves increased to 350 °C compared with that of the native film (320 °C). In addition, the TGA patterns of the Gel–MCC composites exhibited serious phase separation. The mechanical properties changed to some degree, with higher Eab and lower Em, which suggested better flexibility and shatter-proofing. The contact angles, with a highest value of 135.5°, indicated good hydrophobic properties. The swelling ability after absorbing water could be regulated by adding different weights of crosslinker. The light barrier performance was improved with the introduction of MEN compared with both pure gelatin film and Gel/MCC composites. Given the potential applications of gelatin, our study is an extension of the existing NHS crosslinking technique and will broaden the application of gelatin films as a sustained-released material in the food industry, medicine, agriculture, and so on.Acknowledgements
We gratefully acknowledge the promotive research fund for young and middle-aged scientists of Shandong Province (BS2014NJ012), the National Natural Science Foundation of China (no. 21276149) and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.References
- L. B. Guan and W. H. Dan, et al., Gelatin and its Application in Biomedical Material, Mater. Rev., 2006, 20, 380–383 Search PubMed.
- D. Narayanan, M. G. Geena, H. Lakshmi, M. Koyakutty, S. Nair and D. Menon, Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug Ibuprofen-Sodium: An in vitro and in vivo analysis, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9, 818–828 CrossRef CAS PubMed.
- Y. Lu, The research progress of gelatin microspheres, Sci. Technol. Gelatin, 2006, 26(2), 57–70 Search PubMed.
- Y. Lu, The study development of gelatin microballoon sphere, Sci. Technol. Gelatin, 2006, 26(3), 113–134 Search PubMed.
- A. P. Rokhade, S. A. Agnihotri, S. A. Patil, N. N. Mallikarjuna, P. V. Kulkarni and T. M. Aminabhavi, Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine, Carbohydr. Polym., 2011, 65, 243–252 CrossRef PubMed.
- B. D. Kevadiya, S. Rajkumar, H. C. Bajaj, S. S. Chettiar, K. Gosai, H. Brahmbhatt, A. S. Bhatt, Y. K. Barvaliya, G. S. Dave and R. K. Kothari, Biodegradable gelatin–ciprofloxacin–montmorillonite composite hydrogels for controlled drug release and wound dressing application, Colloids Surf., B, 2014, 122, 175–183 CrossRef CAS PubMed.
- P. V. Kozlov and G. I. Burdygina, The structure and properties of solid gelatin and the principles of their modification, Polym. Rev., 1983, 24, 651–666 CrossRef CAS.
- Y. Cao, K. Huang, R. Wu, B. Wang, H. Liu, S. Huang, Y. He and W. Li, Characteristics, Applications and Market Prospect of Microcrystalline Cellulose, Enterprise Science and Technology & Development, 2009, 12, 48–51 Search PubMed.
- A. Casas, S. Omar, J. Palomar, M. Oliet, M. V. Alonsoa and F. Rodriguez, Relation between differential solubility of cellulose and lignin in ionic liquids and activity coefficients, RSC Adv., 2013, 3, 3453–3460 RSC.
- Y. Wu, X. Zhang, B. Lia and S. Liu, Highly transparent and flexible silica/cellulose films with a low coefficient of thermal expansion, RSC Adv., 2014, 4, 52349–52356 RSC.
- O. Gordobil, I. Egüés, I. Urruzola and J. Labidi, Xylan-cellulose films: Improvement of hydrophobicity, thermal andmechanical propertiesOihana, Carbohydr. Polym., 2014, 112, 56–62 CrossRef CAS PubMed.
- Y. Numanoğlu and S. Sungur, β-Galactosidase from Kluyveromyces lactis cell disruption and enzyme immobilization using a cellulose–gelatin carrier system, Process Biochem., 2004, 39, 703–709 CrossRef.
- S.-T. Chang, Li-C. Chen, S.-B. Lin and H.-H. Chen, Nano-biomaterials application: Morphology and physical properties of bacterial cellulose/gelatin composites via crosslinking [J], Food Hydrocolloids, 2012, 27, 137–144 CrossRef CAS PubMed.
- S. Qin, EDTA Dianhydride and Their Derivatives, Chem. Res. Appl., 1993, 5(4), 1–13 Search PubMed.
- Y. Xing, D. Liu and Li-P. Zhang, Enhanced adsorption of Methylene Blue by EDTAD-modified sugarcane bagasse and photocatalytic regeneration of the adsorbent, Desalination, 2010, 259, 187–191 CrossRef CAS PubMed.
- Y. Luo, H. Peng, J. Wu, J. Sun and Y. Wang, Novel amphoteric pH-sensitive hydrogels derived from ethylenediaminetetraacetic dianhydride, butanediamine and amino-terminated poly(ethylene glycol): Design, synthesis and swelling behavior, Eur. Polym. J., 2011, 47, 40–47 CrossRef CAS PubMed.
- C. Yang, L. Xua, Y. Zhou, X. Zhang, X. Huang, M. Wang, Y. Han, M. Zhai, S. Wei and J. Li, A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing, Carbohydr. Polym., 2010, 82, 1297–1305 CrossRef CAS PubMed.
- C. Abrusci, D. Marquina, A. Del Amo and F. Catalina, Biodegradation of cinematographic gelatin emulsion by bacteria and filamentous fungi using indirect impedance technique, Int. Biodeterior. Biodegrad., 2007, 60, 137–143 CrossRef CAS PubMed.
- J. F. Martucci and R. A. Ruseckaite, Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions, Polym. Degrad. Stab., 2009, 94, 1307–1313 CrossRef CAS PubMed.
- S. Lotfy and Y. H. A. Fawzy, Characterization and enhancement of the electrical performance of radiation modified poly(vinyl) alcohol/gelatin copolymer films doped with carotene, J. Radiat. Res. Appl. Sci., 2014, 7, 338–345 CrossRef PubMed.
- N. Ninan, Y. Grohens, A. Elain, N. Kalarikkal and S. Thomas, Synthesis and characterisation of gelatin/zeolite porous scaffold, Eur. Polym. J., 2013, 49, 2433–2445 CrossRef CAS PubMed.
- E. Chiellinia, P. Cinelli, A. Corti and El R. Kenawy, Composite films based on waste gelatin: thermal–mechanical properties and biodegradation testing, Polym. Degrad. Stab., 2001, 73, 549–555 CrossRef.
- M. Jridi, S. Hajji, H. B. Ayed, I. Lassoued, A. Mbarek, M. Kammoun, N. Souissi and M. Nasri, Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films, Int. J. Biol. Macromol., 2014, 67, 373–379 CrossRef CAS PubMed.
- J.-H. Li, J. Miao, J.-L. Wu, S.-F. Chen and Qi-Q. Zhang, Preparation and characterization of active gelatin-based films incorporated with natural antioxidants, Food Hydrocolloids, 2014, 37, 166–173 CrossRef CAS PubMed.
- J. S. Alves, K. C. dos Reis, E. G. T. Menezes, F. V. Pereira and J. Pereira, Effect of cellulose nanocrystals and gelatin in corn starch plasticizedfilms, Carbohydr. Polym., 2015, 115, 215–222 CrossRef CAS PubMed.
- R. Andrade, O. Skurtys, F. Osorio, R. Zuluaga, P. Gañán and C. Castro, Wettability of gelatin coating formulations containing cellulose nanofibers on banana and eggplant epicarps, LWT–Food Sci. Technol., 2014, 58, 158–165 CrossRef CAS PubMed.
- Y. Cheng, J. Lua, S. Liu, P. Zhao, G. Lu and J. Chen, The preparation, characterization and evaluation of regeneratedcellulose/collagen composite hydrogel films, Carbohydr. Polym., 2014, 107, 57–64 CrossRef CAS PubMed.
- C. Mu, J. Guo, X. Li, W. Lin and D. Li, Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films, Food Hydrocolloids, 2012, 27, 22–29 CrossRef CAS PubMed.
- M. Zhang, K. Wu and G. Y. Li, Interactions of collagen molecules in the presence of N-hydroxysuccinimide activated adipic acid (NHS-AA) as a crosslinking agent, Int. J. Biol. Macromol., 2011, 49, 847–854 CrossRef CAS PubMed.
- T. Kajiyama, H. Kobayashi, T. Taguchi, H. Saitoc, Y. Kamatsua, K. Kataoka and J. Tanaka, Synthesis of activated poly (α,β-malic acid) using N-hydroxysuccinimide and its gelation with collagen as biomaterials, Mater. Sci. Eng., C, 2004, 24, 815–819 CrossRef PubMed.
- H. Saito, T. Taguchi, H. Aoki, S. Murabayashi, Y. Mitamura, J. Tanaka and T. Tateishi, PH-responsive swelling behavior of collagen gels prepared by novel crosslinkers based on naturally derived di- or tricarboxylic acids, Acta Biomater., 2007, 3, 89–94 CrossRef CAS PubMed.
- O. Karnitz Jr, L. Vinícius, A. Gurgel, R. P. de Freitas and L. F. Gil, Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD), Carbohydr. Polym., 2009, 77, 643–650 CrossRef PubMed.
- O. Karnitz Jr, L. Vinicius, A. Gurgel, J. C. P. de Melo, V. R. Botaro, T. M. S. Melo, R. P. de Freitas Gil and L. F. Gil, Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse, Bioresour. Technol., 2007, 98, 1291–1297 CrossRef PubMed.
- K. A. G. Gusmão, L. V. A. Gurgel, T. M. S. Melo and L. F. Gil, Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride (EDTAD) in aqueous solutions: Kinetic and equilibrium aspects, J. Environ. Manage., 2013, 118, 135–143 CrossRef PubMed.
- O. Karnitz Jr, L. V. A. Gurgel and L. F. Gil, Removal of Ca(II) and Mg(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse grafted with EDTA dianhydride (EDTAD), Carbohydr. Polym., 2010, 79, 184–191 CrossRef PubMed.
- Y. H. Chen, M. Zhang, W. T. Liu and G. Y. Li, Properties of alkali-solubilized collagen solution crosslinked by N-hydroxysuccinimide activated adipic acid, Korea Aust. Rheol. J., 2011, 23, 41–48 CrossRef PubMed.
- D. D. Van Slyke, A method for quantitative determination of aliphatic amino groups: applications to the study of proteolysis and proteolytic products, Biol. Chem., 1911, 9, 185–204 CAS.
- L. D. Li, Quantometer of amino [P]. China. 201220241965.6. 2012-05-28.
- A. A. Haroun, E. F. Ahmed and M. A. Abd El-Ghaffar, Preparation and antimicrobial activity of poly(vinylchloride)/gelatin/montmorillonite biocomposite films, J. Mater. Sci.: Mater. Med., 2011, 22, 2545–2553 CrossRef CAS PubMed.
- P. Tongnuanchan, S. Benjakul and T. Prodpran, Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils, Food Chem., 2012, 134(3), 1571–1579 CrossRef CAS PubMed.
- Y. Fang, M. A. Tung, I. J. Britt, S. Yada and D. G. Dalgleish, Tensile and Barrier Properties of Edible Films Made from Whey Proteins, J. Food Sci., 2002, 67, 188–193 CrossRef CAS PubMed.
- G. Kavoosi, A. Rahmatollahi, S. M. M. Dadfar and A. M. Purfard, Effects of essential oil on the water binding capacity, physicomechanical properties, antioxidant and antibacterial activity of gelatin films, LWT–Food Sci. Technol., 2014, 57, 556–561 CrossRef CAS PubMed.
- X. Zheng, J. Liu, Y. Pei, J. Li and K. Tang, Preparation and properties of sisal microfibril/gelatin biomass composites, Composites, Part A, 2012, 43, 45–52 CrossRef PubMed.
- K. Watanabe, M. Tabuchi, Y. Morinaga and F. Yoshinaga, Structural features and properties of bacterial cellulose produced in agitated culture, Cellulose, 1998, 5, 187–200 CrossRef CAS.
- C. Seok Ki, D. H. Baek, K. D. Gang, Ki H. Lee, In C. Um and Y. H. Park, Characterization of gelatin nanofiber prepared from gelatin–formic acid solution, Polymer, 2005, 46, 5094–5102 CrossRef PubMed.
- J. Wang, Y. Z. Wan, H. L. Luo, C. Gao and Y. Huang, Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique, Mater. Sci. Eng., C, 2012, 32, 536–541 CrossRef CAS PubMed.
- J. Xu, T. D. Li, X. L. Tang, C. D. Qiao and Q. W. Jiang, Effect of aggregation behavior of gelatin in aqueous solution on the grafting density of gelatin modified with glycidol [J], Colloids Surf., B, 2012, 95, 201–207 CrossRef CAS PubMed.
- N. Roy, N. Saha, T. Kitano and P. Saha, Biodegradation of PVP-CMC hydrogel film: A useful food packaging material, Carbohydr. Polym., 2012, 89, 346–353 CrossRef CAS PubMed.
- D. Shen, R. Xiao, S. Gu and K. Luo, The pyrolytic behavior of cellulose in lignocellulosic biomass: a review, RSC Adv., 2011, 1, 1641–1660 RSC.
- T. M. Santos, M. de S. á M. S. Filho, C. A. Caceres, M. F. Rosa, J. P. S. Morais, A. M. B. Pinto and H. M. C. Azeredo, Fish gelatin films as affected by cellulose whiskers and sonication, Food Hydrocolloids, 2014, 41, 113–118 CrossRef CAS PubMed.
- J. F. Martucci and R. A. Ruseckaite, Tensile properties, barrier properties, and biodegradation in soil of compression-molded gelatin-dialdehyde starch films, J. Appl. Polym. Sci., 2009, 112, 2166–2178 CrossRef CAS PubMed.
- A. Bigi, G. Cojazzi, S. Panzavolta, K. Rubini and N. Roveri, Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking, Biomaterials, 2001, 22, 763–768 CrossRef CAS.
- L. S. Matsuguma, L. G. Lacerda, E. Schnitzler, M. A. D. Carvalho Filho, S. Franco and C. M. L. I. M. Demiate, Characterization of native and oxidized starches of two varieties of Peruvian carrot (Arracacia xanthorrhiza, B.) from two production areas of Paraná state, Brazil, Braz. Arch. Biol. Technol., 2009, 52, 701–713 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04808g |
| This journal is © The Royal Society of Chemistry 2015 |