Insulin templated synthesis of single-crystalline silver nanocables with ultrathin Ag cores†
Abstract
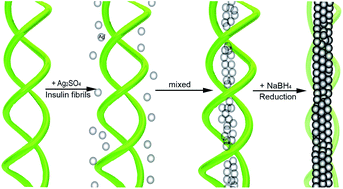
The synthesis of ultrathin single-crystal 1D (one dimensional) nanostructures is highly desirable for potential applications as nanoconnectors and nanoscale devices. In this work, we demonstrate the preparation of straight single-crystalline coaxial silver nanocables (13 nm core diameter, 1.5 nm sheath thickness) in large amounts with excellent dispersibility for the first time through a self assembly approach via insulin fibril templates. This outstanding method for obtaining silver nanocables with ultrathin diameters will be attractive for the fabrication of other metallic 1D nanostructures, and this study may offer an effective strategy to design a novel silver nanostructure with diverse functionalities and potential applications in conductive materials, sensors and catalysts.

 Please wait while we load your content...
Please wait while we load your content...