Effects of rapid thermal annealing on the properties of AlN films deposited by PEALD on AlGaN/GaN heterostructures
Abstract
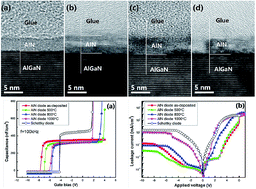
Aluminum nitride (AlN) films have been deposited on AlGaN/GaN heterostructure substrates by plasma enhanced atomic layer deposition (PEALD). Different annealing treatments were adopted to change film structure and improve performance. Chemical composition, crystallinity, and electrical properties were studied for AlN films. The results show that some crystal grains appear in the films after annealing at a temperature of over 800 °C. The film crystalline quality increases as the annealing temperature rises. The N–O–Al bond decomposes during the high temperature annealing in N2, and some new N–Al bonds are formed in the AlN films. Annealing promotes the elemental interdiffusion between the films and the substrates. High-temperature annealing at 1000 °C in a nitrogen atmosphere can effectively promote complete nitridation of the AlN film, reduce the nitrogen vacancies, and cause the AlN film to form a semiconductor-like structure.

 Please wait while we load your content...
Please wait while we load your content...