Discovery of 4-benzoylpiperidine and 3-(piperidin-4-yl)benzo[d]isoxazole derivatives as potential and selective GlyT1 inhibitors†
Yang Liua,
Lin Guob,
Hongliang Duana,
Liming Zhanga,
Neng Jiangc,
Xuechu Zhen*b and
Jianhua Shen*a
aState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: jhshen@mail.shcnc.ac.cn
bJiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases and Department of Pharmacology, Soochow University, Suzhou 215006, China. E-mail: zhenxuechu@suda.edu.cn
cChina Pharmaceutical University, Nanjing 210009, China
First published on 30th April 2015
Abstract
Regulation of glycine transporter 1 (GlyT1) activity is a currently investigated strategy in drug discovery for schizophrenia. This study developed a series of new 4-benzoylpiperidine derivatives as GlyT1 inhibitors by bioisosteric replacement and mimicking of the pyridine ring of RG1678. Among the 4-benzoylpiperidine derivatives, 23q showed an IC50 of 30 nM. Preliminary optimization of the blood–brain barrier penetration led to the discovery of 3-(piperidin-4-yl)benzo[d]isoxazole derivatives. Both series showed good selectivity over GlyT2, D1, D2, D3, 5-HT1A and 5-HT2A receptors. Moreover, behavioral testing showed 23q (40 mg kg−1, intragastric) can inhibit the hyperlocomotion induced by acute treatment of phencyclidine, and improve the impaired negative and cognitive symptoms in chronic phencyclidine-induced C57BL/6J mice. An interesting finding showed that 3-(piperidin-4-yl)benzo[d]isoxazole was a privileged scaffold of atypical antipsychotic agents but exhibited high selectivity and potency as a GlyT1 inhibitor.
Introduction
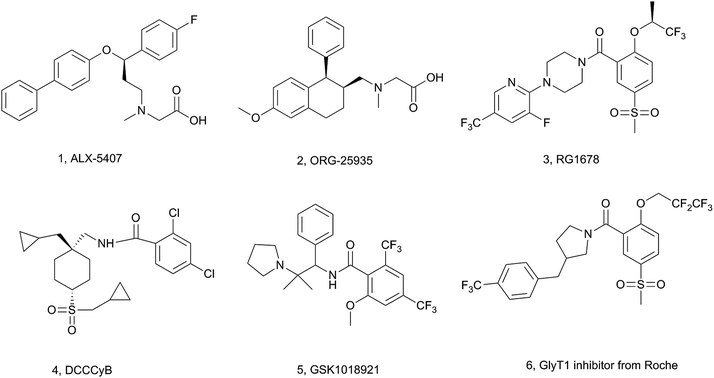
Schizophrenia is a severe and chronic psychiatric illness, characterized by positive, negative and cognitive symptoms. Currently available drugs are effective in relief of the positive symptoms, but elicit weak or no improvement in the negative symptoms and cognitive impairments.1 It is generally believed that the N-methyl-D-aspartate (NMDA) receptor hypofunction plays a critical role in the pathophysiology of schizophrenia and may associate with the development of negative symptoms and cognitive deficits in the illness.2 Activating the NMDA receptor and restoring function of glutamatergic neurons may be an alternative therapeutic strategy for schizophrenia treatment.3 Because the NMDA receptor is widely distributed in the brain and direct activation of the NMDA receptor would cause seizures and serious neurotoxic side effects,4 efforts have turned to the auxiliary glycine binding site. Glycine is an obligatory co-agonist of the NMDA receptor, and elevated glycine levels in the synaptic cleft can promote activation of the NMDA receptor.5 Glycine in the synaptic cleft can be uptaken into neurons or surrounding glia by the glycine transporter (GlyT). There are two types of glycine transporters in the brain: GlyT1, which is localized to the NMDA receptor area, and GlyT2, which is distributed around the glycinergic neurons.6 Therefore, selective inhibition of GlyT1 could elevate glycine concentrations in the glutamatergic synapse and enhance NMDA receptor activity, thereby may be of potential therapeutic effects in schizophrenia.So far, at least two series of GlyT1 inhibitors have been identified (Fig. 1).7 One is the sarcosine-derived GlyT1 inhibitor, such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 2.9 However, some compounds of this series were discontinued in clinical trials because of undesired adverse effects. Another series is the non-sarcosine-based GlyT1 inhibitor, such as 3,10 4,11 and 5.12 Among them, RG1678 demonstrated a beneficial effect in patients with schizophrenia characterized with predominant negative symptoms in a phase II proof-of-concept study13 and it is currently in phase III clinical trial.
8 and 2.9 However, some compounds of this series were discontinued in clinical trials because of undesired adverse effects. Another series is the non-sarcosine-based GlyT1 inhibitor, such as 3,10 4,11 and 5.12 Among them, RG1678 demonstrated a beneficial effect in patients with schizophrenia characterized with predominant negative symptoms in a phase II proof-of-concept study13 and it is currently in phase III clinical trial.
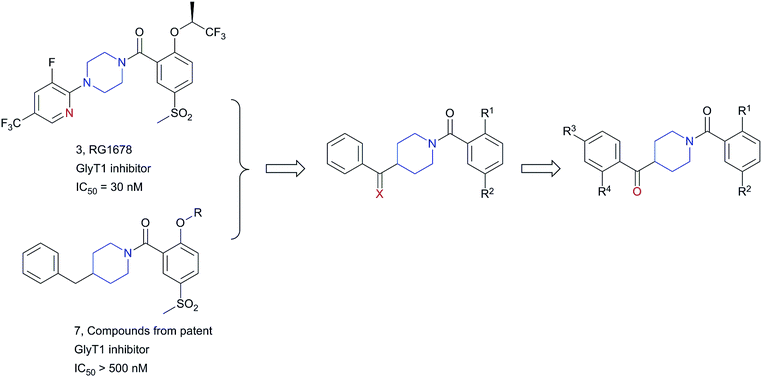
In our effort to find new GlyT1 inhibitors, we considered RG1678 as a potential starting point for further structural modification. A patent from Roche revealed some bioisosteric replacements of piperazine ring, among them pyrrolidine series was the most potent and well investigated (compound 6 with an IC50 = 90 nM) while piperidine ring did not exhibit good potency.14 In this case, we considered that piperidine ring was an appropriate bioisostere of piperazine and when the piperazine ring was replaced with piperidine, some heteroatoms should be incorporated to compensate for the loss of binding energy like hydrogen bond. Based on this consideration, we first introduced a carbonyl to 4-benzylpiperidine of compound 7 and a series of 4-benzoylpiperidine derivatives were synthesized, and tested (Fig. 2).
Results and discussion
Chemistry
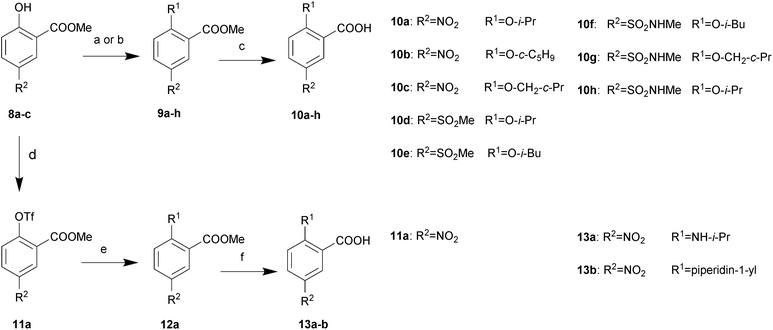
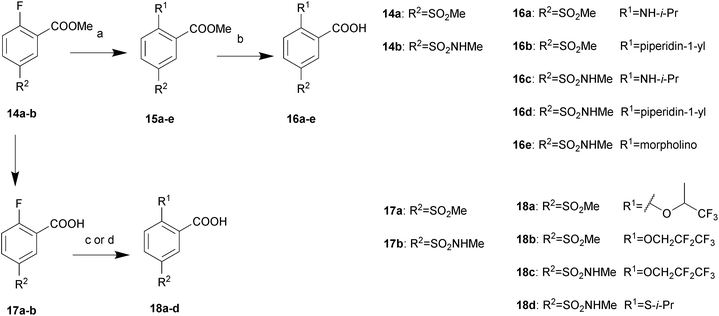
All described target compounds were prepared by condensation of 2,5-disubstituted benzoic acids and corresponding piperidine moieties. The 2,5-disubstituted benzoic acids were performed as shown in Schemes 1 and 2. 8a–c were commercially available or synthesized as listed in ref. 10. The R2 group preferred an electron-withdrawing groups (EWGs). So it was selected from nitro, methylsulfonyl, and N-methylsulfamoyl. First, 2-alkoxy-5-substituted benzoates 9a–h were prepared by substitution reactions with alkyl halides or Mitsunobu reactions with alkyl alcohols in good yields. Then the substituted esters were hydrolyzed by sodium hydroxide to afford the corresponding acids 10a–h. By reacting with triflic anhydride, the phenolic hydroxyl group of 8a–b was converted into trifluoromethylsulfonyloxy group in 72–79% yields and then coupled with 4-fluorophenylboronic acid or substituted by some alkyl amines to yield 12a–b. Further hydrolysis was performed to get 13a–b. Alternatively, considering the electron-withdrawing groups of the phenyl ring, the alkoxy and alkyl amine group at position 2 was also introduced by 2-fluorobenzoic acid analogues following the synthetic route described in Scheme 2.The synthesis of piperidine moieties is outlined in Scheme 3. Substituted bromobenzene was converted to a Grignard reagent and then reacted with Weinreb amide, resulting in 20a–b. Following reflux in HCl (6 N), 21a–b were prepared. 21c was synthesized according to a previous study15 and 21d was commercially available. Finally, the target compounds were prepared in a single step by HATU-mediated condensation yields ranging from 57% to 85%.
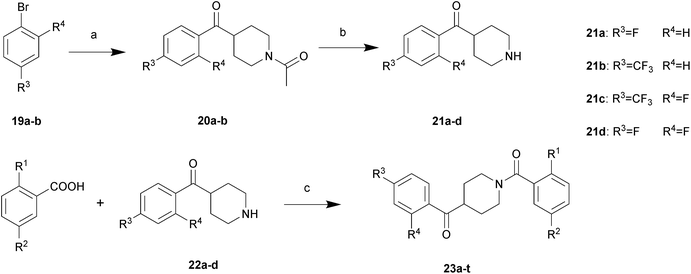 | ||
| Scheme 3 Reagents and conditions: (a) Mg, I2, dry THF, 1-acetyl-N-methoxy-N-methylpiperidine-4-carboxamide, reflux to rt, overnight; (b) HCl (6 N), reflux, 4 h; (c) HATU, Et3N, CH2Cl2, rt, overnight. | ||
In vitro biological evaluation and discussion
All final compounds were initially evaluated for their ability to inhibit GlyT1, and the results are summarized in Tables 1 and 2. Considering the convenience of synthesis, we introduced a nitro group to R2 position first and then explored the R3 and R4 group. The structure–activity relationship study (SAR) of RG1678 revealed that the aromatic ring of the left side preferred hydrophobic substituents, so we incorporated trifluoromethyl and fluoro to R3 and R4 positions. As shown in Table 1, for the R4 group, fluoro was more potent than hydrogen (23a vs. 23b, 23c vs. 23d, and 23e vs. 23f), while on the R3 position, trifluoromethyl was less suitable than fluoro (23a vs. 23c and 23b vs. 23d). For the R1 group, introduction of some alkylaminos, such as 23g and 23h, led to great loss in GlyT1 activity. Though 23e showed a slight increase of activity against 7, the others did not meet our satisfaction. This result prompted us to optimize the substituents of the benzoic moiety.| Compd | R1 | R2 | R3 | R4 | GlyT1 IC50 (nM)a |
|---|---|---|---|---|---|
| a IC50 values were determined using [3H] glycine and rat glioma C6 cell lines. Data are the mean ± SEM of at least three independent determinations. | |||||
| 23a | 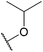 |
Nitro | F | F | 471 ± 54 |
| 23b | 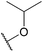 |
Nitro | F | H | 2790 ± 40 |
| 23c | 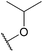 |
Nitro | CF3 | F | 1855 ± 45 |
| 23d |  |
Nitro | CF3 | H | 77.87%@10 μM |
| 23e |  |
Nitro | F | F | 310 ± 94 |
| 23f |  |
Nitro | F | H | 461 ± 165 |
| 23g |  |
Nitro | F | F | 78.53%@10 μM |
| 23h |  |
Nitro | F | F | 82.45%@10 μM |
| Compd | R1 | R2 | GlyT1 IC50a (nM) |
|---|---|---|---|
| a IC50 values were determined using [3H] glycine and rat glioma C6 cell lines. Data are the mean ± SEM of at least three independent determinations. | |||
| 23i |  |
N-Methylsulfamoyl | 172 ± 36 |
| 23j | 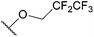 |
N-Methylsulfamoyl | 143 ± 7.25 |
| 23k |  |
N-Methylsulfamoyl | 117 ± 5.37 |
| 23l |  |
N-Methylsulfamoyl | 184 ± 14 |
| 23m |  |
N-Methylsulfamoyl | 80 ± 10.47 |
| 23n |  |
N-Methylsulfamoyl | 115 ± 32.9 |
| 23o |  |
Methylsulfonyl | 58.02%@10 μM |
| 23p | 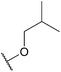 |
Methylsulfonyl | 501 ± 84.9 |
| 23q |  |
Methylsulfonyl | 30 ± 5 |
| 23r |  |
Methylsulfonyl | 572 ± 67 |
| 23s |  |
Methylsulfonyl | 44.73%@10 μM |
| 23t |  |
Methylsulfonyl | 955 ± 101 |
As shown in Table 2, nitro was first replaced with another electron withdrawing N-methylsulfamoyl group while R3 and R4 were kept as the most potent fluoro. To our delight, the N-methylsulfamoyl series exhibited well tolerance on the R1 group, and all alkoxy and alkylamino groups on R1 position showed moderate activities. However, none of the compounds had a better performance with an IC50 value below 50 nM. Finally, we tested methylsulfonyl on R2 position of benzoic moiety. The result was consistent with nitro series and alkoxy groups were more potent than alkylamino on R1 position. Surprisingly, 23q gave the most satisfactory result (IC50 = 30 nM). The various substituents at R1 position of benzoic that were tolerated indicated that a lipophilic bulk pocket might exist at this position for the GlyT1 receptor.
In vivo pharmacokinetic study
With the identification of 23q as the most potent GlyT1 inhibitor in vitro, the pharmacokinetic parameters of 23q were evaluated in ICR mice. After oral administration of 23q (10 mg kg−1), blood samples and brain tissues were collected and analyzed at different time points. As shown in Table 3, 23q had a short half-life (T1/2 = 0.7 h), moderate plasma exposure (AUC0–∞ = 737 h ng mL−1) with a low brain exposure (Cmax in brain = 26.3 ng g−1), indicating a poor B/P ratio. However, the concentration in the brain (Cmax in brain = 26.3 ng g−1) was just above the IC50 value. This result promoted us to make structure modifications to improve the brain exposure.| Compd | T1/2 (h) | Tmax (h) | Cmax,pa (ng mL−1) | AUC0–t (h ng mL−1) | AUC0–∞ (h ng mL−1) | MRT0–∞ (h) | Cmax,bb (ng g−1) | B/Pc |
|---|---|---|---|---|---|---|---|---|
| a Cmax,p = Cmax in plasma.b Cmax,b = Cmax in brain.c B/P = Cmax,b/Cmax,p. n = 3 mice per group; male ICR mice (18–20 g); 10 mg kg−1, intragastric administration, formulated in 0.5% HMPC. PK parameters were calculated by non-compartmental model analysis on Phoenix WinNonlin 6.0 (Pharsight, Mountain View, CA). | ||||||||
| 23q | 0.7 | 0.5 | 955 | 728 | 737 | 0.91 | 26.3 | 0.03 |
Initial attempts to improve brain exposure
The low B/P ratio of 23q indicated that it may be difficult to penetrate the Blood–Brain Barrier (BBB). Strategies to improve BBB permeability have been reviewed and the structure optimization methods include increasing lipophilicity, reducing hydrogen bond donors, increasing rigidity and lowering polar surface area (PSA).16 Herein we used two parameters, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and tPSA (topological PSA) calculated by ChembioDraw, to represent the lipophilicity and PSA.
P and tPSA (topological PSA) calculated by ChembioDraw, to represent the lipophilicity and PSA.
As shown in Table 4, 23q had a similar tPSA value with RG1678 while a slightly lower C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, which indicating that higher lipophilicity may be helpful to improve the B/P ratio. At the same time, we made a confirmation restriction of 4-benzoylpiperidine moiety, leading to the 3-(piperidin-4-yl)benzo[d]isoxazole derivatives. By this transformation, we kept the heteroatom in proper position, increased the rigidity and lipophilicity of molecule, but caused a slight adverse effect to tPSA. When 23q was transformed to 24a, a great loss in activity was also observed (IC50 = 185 nM). A scale of optimization was performed and the selected compounds summarized in Table 4 demonstrated that 3-(piperidin-4-yl)benzo[d]isoxazole derivatives had moderate to acceptable GlyT1 inhibiting activities (24g with an IC50 = 38 nM). But the initial optimization failed to reach a balance among the activity, C
P, which indicating that higher lipophilicity may be helpful to improve the B/P ratio. At the same time, we made a confirmation restriction of 4-benzoylpiperidine moiety, leading to the 3-(piperidin-4-yl)benzo[d]isoxazole derivatives. By this transformation, we kept the heteroatom in proper position, increased the rigidity and lipophilicity of molecule, but caused a slight adverse effect to tPSA. When 23q was transformed to 24a, a great loss in activity was also observed (IC50 = 185 nM). A scale of optimization was performed and the selected compounds summarized in Table 4 demonstrated that 3-(piperidin-4-yl)benzo[d]isoxazole derivatives had moderate to acceptable GlyT1 inhibiting activities (24g with an IC50 = 38 nM). But the initial optimization failed to reach a balance among the activity, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and tPSA. As we expected, with a higher tPSA value, 24g did not have better performance than 23q in vivo pharmacokinetic study (data not shown).
P and tPSA. As we expected, with a higher tPSA value, 24g did not have better performance than 23q in vivo pharmacokinetic study (data not shown).
| Compd | R1 | R2 | GlyT1 IC50a (nM) | C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb Pb |
tPSAc |
|---|---|---|---|---|---|
| a IC50 values were determined using [3H] glycine and rat glioma C6 cell lines. Data are the mean ± SEM of at least three independent determinations.b Data were calculated by ChemBioDraw.c Data were calculated by ChemBioDraw.d Data was selected from published reference. | |||||
| RG1678 | — | — | 30d | 2.99 | 79.28 |
| 23q | — | — | 30 ± 5 | 2.67 | 80.75 |
| 24a |  |
Methylsulfonyl | 185 ± 56 | 3.28 | 85.27 |
| 24b | 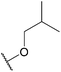 |
Methylsulfonyl | 159 ± 43 | 2.93 | 85.27 |
| 24c |  |
Methylsulfonyl | 496 ± 58.9 | 2.58 | 85.27 |
| 24d |  |
Nitro | 89 ± 7.8 | 4.19 | 102.94 |
| 24e |  |
Nitro | 85 ± 5.15 | 2.46 | 79.28 |
| 24f |  |
N-Methylsulfamoyl | 61 ± 6.83 | 2.92 | 97.3 |
| 24g |  |
N-Methylsulfamoyl | 38 ± 7.63 | 3.54 | 97.3 |
Selectivity evaluation of 23 and 24 series
Though 24 series was unsuccessful to improve the B/P ratio, we were surprised to find that the 3-(piperidin-4-yl)benzo[d]isoxazole moiety was a privileged scaffold that appears frequently in atypical antipsychotic agents17 such as risperidone18 and iloperidone,19 which were dopamine and serotonin receptors dual antagonists. 4-Benzoylpiperidine moiety was also incorporated to discover multitarget ligands of aminergic receptors.20 With this in mind, we assessed the selectivity of some selected compounds. Data were shown in Table 5. Both 23 and 24 series exhibited high selectivity over GlyT2, dopamine and serotonin receptors (IC50 > 10 μM), indicating that 23 and 24 series were selective GlyT1 inhibitors. On the other hand, the result demonstrated that 3-(piperidin-4-yl)benzo[d]isoxazole moiety was not only a privileged scaffold in dopamine hypothesis, but also well tolerated in NMDA hypofunction hypothesis.| Compd | GlyT1 IC50 (nM) | GlyT2a @10 μM | D1b @10 μM | D2b @10 μM | D3b @10 μM | 5-HT1Ab@10 μM | 5-HT2Ab @10 μM | hERGc IC50 (μM) |
|---|---|---|---|---|---|---|---|---|
| a GlyT2 inhibiting activity was examined with the cultured primary brain stem neurons.b D1, D2, D3, 5-HT1A, and 5-HT2A were obtained from HEK293 stable-transfecting cell lines.c hERG channels were obtained from HEK293 stable-transfecting cell lines.d Not tested. | ||||||||
| 23i | 172 ± 36 | 25.67% | 19.73% | 5.87% | 25.14% | 2.90% | 0.66% | —d |
| 23k | 117 ± 5.37 | 11.94% | 7.62% | 18.45% | 14.43% | 33.06% | 8.84% | — |
| 23m | 80 ± 10.47 | 14.92% | 14.87% | 6.20% | 10.80% | 5.80% | 4.86% | — |
| 23q | 30 ± 5 | 14.62% | 16.33% | 10.31% | 15.29% | 11.22% | 5.21% | 8.1 |
| 24b | 159 ± 43 | 8.65% | 22.25% | 24.24% | 17.49% | 3.91% | 4.05% | — |
| 24d | 89 ± 7.8 | 18.95% | 23.37% | 5.20% | 21.51% | 1.75% | 31.55% | — |
| 24f | 61 ± 6.83 | 19.70% | 6.21% | 3.94% | 30.30% | 24.01% | 6.39% | — |
| 24g | 38 ± 7.63 | 17.61% | 8.02% | 22.48% | 25.81% | 35.67% | 16.51% | — |
In vivo behavioral tests on C57BL/6J mice
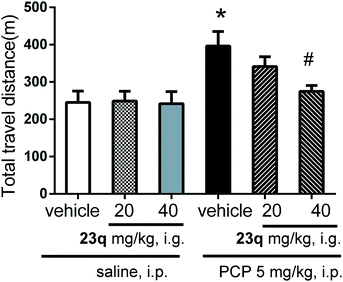
With the identification of 23q as a potent and selective GlyT1 inhibitor, we assessed in vivo anti-psychotic activity using phencyclidine (PCP)-induced schizophrenia-like animal models. Considering the low B/P ratio, we assessed using higher doses (20, 40 mg kg−1, intragastric).Firstly, the effect of 23q on the acute PCP-induced hyperlocomotor activity was examined as described previously,21 23q alone did not change the mice's locomotor activity. Pretreatment of 23q (40 mg kg−1) attenuated PCP (5 mg kg−1)-induced hyperlocomotion of mice, indicating that 23q is a potential anti-psychotic drug in vivo (Fig. 3).
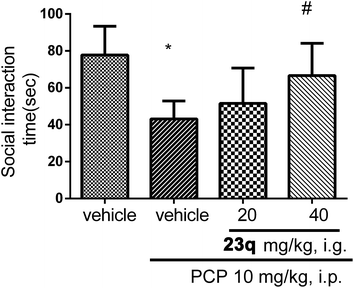
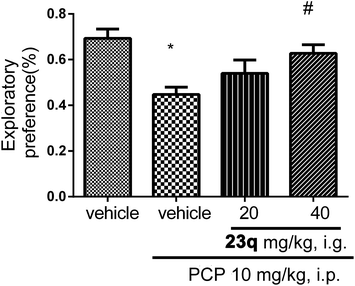
We then examined whether 23q could improve the social interaction and cognitive function. C57BL/6J mice received daily injections of PCP (10 mg kg−1) for 2 weeks prior to administration of 23q for an additional 2 weeks. Then social interaction and novel object recognition tests were performed. Methods are summarized in the Experimental section.21–23
As shown in Fig. 4, mice treated with PCP exhibited a social behavioral deficit in the social interaction test. Two weeks of treatment with 23q (40 mg kg−1) significantly restored the impaired social interaction in PCP-treated mice. Similarly, administration of 23q (40 mg kg−1) significantly improved the cognitive deficit in the chronic PCP-treated mice (Fig. 5).
Conclusions
In summary, based on the bioisosteric replacement and mimic of nitrogen atom of pyridine ring of RG1678, we synthesized and evaluated a series of 4-benzoylpiperidine derivatives (23a–t). Among them 23q was the most potent GlyT1 inhibitor in vitro with an IC50 value of 30 nM and showed high selectivity against GlyT2 and dopamine and serotonin receptors. Further in vivo study demonstrated that 23q was effective on the chronic PCP-treated schizophrenia-like behavioral models with 40 mg kg−1. The relatively high dose of 23q was needed to achieve the anti-psychotic efficacy may attribute to the poor pharmacokinetic parameters and low B/P ratio. Preliminary optimization did not improve the pharmacokinetic parameters but led to the discovery of another potential and selective GlyT1 inhibitors: the 3-(piperidin-4-yl)benzo[d]isoxazole derivatives. Next we will make more structure modifications to improve the brain exposure. 4-Benzoylpiperidine and 3-(piperidin-4-yl)benzo[d]isoxazole, which were privileged scaffolds in atypical antipsychotic agents, exhibited high potency in GlyT1 inhibition. We suspect whether they may be potential templates to build multitarget ligands of GlyT1, dopamine and serotonin receptors.Experimental section
General
All reagents were purchased from commercial suppliers and used without further purification unless otherwise stated. Yields were not optimized. Microwave reactions were performed in a Biotage Initiator. Column chromatography was performed using pre-packed silica cartridges (from 4 to 40 g) from Bonna-Agela Technologies Inc. (Tianjin, China) and eluted with a CombiFlash® Rf 200 from Teledyne Isco. 1H NMR and 13C NMR spectra was recorded on a Bruker AC300 or a Bruker AC400 NMR spectrometer, using tetramethylsilane as an internal reference. Low-resolution mass spectra were determined on Agilent liquid-chromatography mass spectrometer system that consisted of an Agilent 1260 infinity LC coupled to Agilent 6120 Quadrupole mass spectrometer (electrospray positive ionization; ESI) using an Agilent ZORBAX 1.8 mm SB-C18 column (2.1 × 50 mm) with aqueous CH3CN (30–90%) containing 0.05% formic acid monitored at 240 nm. High-resolution mass spectra were recorded on a Q-Tof Ultima Globe mass spectrometer (Micromass, Manchester, UK). HPLC analysis for all compounds tested in biological systems was performed on an Agilent 1200 series LC system (Agilent ChemStation, Agilent Eclipse XDB-C18, 5 μm, 4.6 × 150 mm, 30 °C, UV240 nm, 1.0 mL min−1) with aqueous CH3CN (35–90%) containing formic acid (0.05%) for 25 min. Experiments in pharmacokinetics study and behavioral tests with live animals were performed in compliance with the Guidelines for the Care and Use of Laboratory Animals (National Research Council, People's Republic of China, 1996). The animal protocols were approved by the Institutional Animal Care and Use Committees of Shanghai Institute of Materia Medica (SIMM) and Soochow University.General synthetic procedure for benzoic acids and piperidine moieties
Preparation of 10a–h, 13a–b, 16a–e, 18a–d and 21a–b was summarized in ESI.†General synthetic procedure for 23a–t, 24a–g
To a solution of benzoic acid moiety (1 equiv.) in CH2Cl2 (15 mL) was added HATU (1.3 equiv.) and the mixture was stirred at room temperature for 30 min. Then Et3N (3 equiv.) and piperidine moiety (1.2 equiv.) were added subsequently and the reaction mixture was stirred at room temperature overnight. The mixture was diluted with CH2Cl2 (10 mL) and washed with water. The organic layer was dried over MgSO4 and concentrated. The residue was purified by flash column chromatography to give the final compounds.[3H] glycine uptake assay (GlyT1)
Rat glioma C6 cells that stably expressed GlyT1 were plated into 24-well culture plates (1 × 106 per well). After 18 h, the culture medium was discarded and HEPES buffer solution (160 μL) was added. Thereafter, tested compounds (20 μL) and 3H-glycine (20 μL; final concentration ranges: 10−10, 10−9, 10−8, 10−7, 10−6, and 10−5 M) were added to a total volume of 200 μL. In the total uptake wells, the tested compounds were replaced with Hank's balanced salt solution (HBSS; 20 μL). NFPS24 (10 μM) was used to determine nonspecific uptake. After a 30 min incubation, HBSS was discarded and the plate was washed twice with phosphate-buffered saline (500 μL). Then, NaOH (100 μL, 2 M) was added to lyse the cells. The lysate activity was examined, and the inhibiting ratio and IC50 values were calculated.[3H] glycine uptake assay (GlyT2)
GlyT2 inhibiting activity was examined using cultured primary brain stem neurons. In brief, brain stems were removed from brains of rat pups. The tissues were minced with a sterile razor blade and digested with 0.05% trypsin (Sigma) and 0.01% Dnase I (Sigma) at 37 °C for 10 min. Then, the tissues were collected by centrifugation for 5 min. After centrifugation, complete culture media Dulbecco's modified Eagle's medium/F12 (3 mL; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Gibco BRL, Gaithersburg, MD, USA) was added, which contained 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin (100 U mL−1), and streptomycin. Thereafter, this tissue was mechanically dissociated into a single-cell suspension. The dissociated cells were plated into 24 plates pre-coated with polylysine (Sigma). At 48 h later, cytosine arabinoside (5 μM) was added to kill the glial cells. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. On the 10th day of culture, the inhibiting activity of GlyT2 was tested. Sarcosine (10−3 M) was added to all wells to block GlyT1 activity, and glycine (10−4 M) was used to determine nonspecific uptake action. The remaining procedures were identical to those in the GlyT1 test.
1; Gibco BRL, Gaithersburg, MD, USA) was added, which contained 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin (100 U mL−1), and streptomycin. Thereafter, this tissue was mechanically dissociated into a single-cell suspension. The dissociated cells were plated into 24 plates pre-coated with polylysine (Sigma). At 48 h later, cytosine arabinoside (5 μM) was added to kill the glial cells. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. On the 10th day of culture, the inhibiting activity of GlyT2 was tested. Sarcosine (10−3 M) was added to all wells to block GlyT1 activity, and glycine (10−4 M) was used to determine nonspecific uptake action. The remaining procedures were identical to those in the GlyT1 test.
Binding assay for dopamine and serotonin receptors
Dopamine receptors 1, 2, and 3 were obtained from HEK293 stable-transfecting cell lines. Binding assays were conducted as described before.25,26 3H-SCH23390 (10 nM) was used for the binding test of D1 receptor, and 3H-spiperone for the D2 or D3 receptor-binding assay. (+)-Butaclamol (final concentration: 10 μM) was used to determine nonspecific binding activity. After incubating 1 h at 30 °C, Tris–HCl (pre-cooled, 3 mL) was added to stop the binding reaction. The receptor-bound isotope was collected with a Whatman GF/B glass fiber filter. Radioactivity on the fiber filter was examined, and the inhibiting ratio was calculated. In the binding test of 5-HT receptor, 3H-8-OH-DPAT (10 nM) was used to test binding of 5-HT1A, and nonspecific binding activity was determined with 5-HT (10 μM). 3H-ketanserin was used for the 5-HT2A test, and spiperone was used for nonspecific binding activity. The remaining procedures were identical to those in the dopamine receptor tests.hERG channel assay
The K+ current by the hERG was recorded using an Axopatch 200 A amplifier at 24–25 °C. HEK293 cells stably expressing hERG channels were held at a resting voltage of −80 mV. Voltage protocols were controlled by pClamp 9.0 software via the DigiData-1322A interface (Axon Instruments, USA). Electrodes (a tip resistance of 3–5 mΩ) were pulled from borosilicate grass pipettes (Sutter Instruments, USA) and filled with a pipette solution consisted of the following (in mM): 140 KCl, 2 MgCl2, 1 CaCl2, 10 HEPES, 10 EGTA, pH 7.4 adjusted with KOH. The test compound was diluted (1 nM–0.1 mM) in extracellular buffer (NaCl 150 mM, KCl 4 mM, CaCl2 1.2 mM, MgCl2 1 mM, HEPES 10 mM, pH 7.4 with NaOH, 300–310 mOsm), and was added directly using a RSC-100 rapid solution changer with a 9-tube head (BioLogic Co, France). After the cells were stabilized and the currents steady, the amplitude and kinetics of IKhERG were recorded. Offline analysis of the peak tail current was performed using pClamp software 9.0. The amplitude and kinetics of IKhERG were recorded for each drug concentration. Concentration-response curves were fitted by nonlinear regression analysis and IC50 values were reported.Pharmacokinetic study
Male ICR mice (body weight: 18–20 g) were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). Animals were fasted for 12 h but free access to water before administration of the compound. All animals were fed in 3 h after administration. Compound was formulated in 0.5% HMPC and administered orally by gavage at a dose of 10 mg kg−1. Blood samples (0.1 mL) were drawn from retrobulbar venous plexus of mice at 0.5, 1, 2, 4, 8 and 24 h postdose (n = 3 mice per group) and stored in heparinized tubes. After centrifugation for 5 min, plasma (50 μL) was added MeOH–ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 200 μL) and centrifuged for 10 min. Supernatant fluid was stored at −20 °C for analysis. Brain tissues were collected at the same time and stored at −20 °C until analysis. Brain tissues were added 10 volumes of MeOH–ACN (1
1, 200 μL) and centrifuged for 10 min. Supernatant fluid was stored at −20 °C for analysis. Brain tissues were collected at the same time and stored at −20 °C until analysis. Brain tissues were added 10 volumes of MeOH–ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After vortex and centrifugation, supernatant fluid was used to analysis. A LC-MS/MS method was used for the quantification of the tested compound. The PK parameters were calculated by non-compartmental model analysis on Phoenix WinNonlin 6.0 (Pharsight, Mountain View, CA).
1). After vortex and centrifugation, supernatant fluid was used to analysis. A LC-MS/MS method was used for the quantification of the tested compound. The PK parameters were calculated by non-compartmental model analysis on Phoenix WinNonlin 6.0 (Pharsight, Mountain View, CA).
Locomotion test
Male C57BL/6J mice were pre-treated with 23q (20, 40 mg kg−1, intragastric) 60 min before the injection of PCP (5 mg kg−1, intraperitoneal). The mice were placed in a Plexiglas open field arena (40 × 40 × 45 cm, Jiliang Co. Ltd., Shanghai, China) connected with a video-based recording system. Automated activity was recorded, and the total travelling distance was calculated.Social interaction test
In this test, C57BL/6J mice received daily injections of either saline or PCP (10 mg kg−1, intraperitoneal) for 2 weeks prior to administration of vehicle control (saline) or compound 23q (20, 40 mg kg−1, intragastric) for an additional 2 weeks. After treatment, the mice were individually placed into an unfamiliar arena (40 × 40 × 45 cm, Jiliang Co., Ltd., Shanghai, China) simultaneously with a weight-matched male mouse. Their behavior was video-recorded for 10 min. The time spent in interaction was defined as sniffing at any part of the partner's body (mainly the anogenital area), grooming, following, crawling over/under, or boxing/wrestling. Interactions were manually scored.Novel object recognition test
Animal treatment was identical to that described in the social interaction test. On the first day, mice were placed into an open field box (40 × 40 × 45 cm) for 5 min and then immediately returned to their home cages. This was repeated on the second day. On the third day, two identical objects were placed into the open-field box and animals were allowed to explore for 5 min. After this training, the box and objects were cleaned with 75% ethanol to avoid possible instinctive odorant cues. On the fourth day, mice were placed back into the same box, a novel object replaced one of the objects used during training, and mice were allowed to explore freely for 5 min. The familiar object and the novel one were different in shape and color, but similar in size. An exploratory preference index, or the ratio of time spent exploring novel object over total time spent exploring both objects, was used to score recognition memory.Abbreviations
| THF | tetrahydrofuran |
| DMF | N,N-dimethylformamide |
| DMA | N,N-dimethylacetamide |
| HATU | 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| DIAD | diisopropyl azodicarboxylate |
| EWG | electron-withdrawing group |
| NFPS | N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine |
| PCP | phencyclidine |
| SAR | structure–activity relationship |
| HPLC | high performance liquid chromatography |
| EGTA | ethylene glycol tetraacetic acid |
| ACN | acetonitrile |
| HMPC | hydroxypropyl methyl cellulose |
| i.g. | intragastric |
| i.p. | intraperitoneal |
Acknowledgements
This work was financially supported by grants from the National Nature Science Foundation of China (grant 81402786, 81130023), National Basic Research Plan (973) of the Ministry of Science and Technology of China (2011CB5C4403). Supports from Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD) and Grant from Jiangsu Science and Technology commission (BY2011131) are also appreciated.Notes and references
- J. T. Coyle, D. Balu, M. Benneyworth, A. Basu and A. Roseman, Dialogues in Clinical Neuroscience, 2010, vol. 12, pp. 359–382 Search PubMed.
- W. Danysz and C. G. Parsons, Pharmacol. Rev., 1998, 50, 597–664 CAS.
- J. Kantrowitz and D. C. Javitt, Current Opinion in Psychiatry, 2012, vol. 25, pp. 96–102 Search PubMed.
- M. J. Marino, L. J. Knutsen and M. Williams, J. Med. Chem., 2008, 51, 1077–1107 CrossRef CAS PubMed.
- C. Sur and G. G. Kinney, Curr. Drug Targets, 2007, 8, 643–649 CrossRef CAS.
- L. Raiteri and M. Raiteri, J. Neurochem., 2010, 114, 647–653 CrossRef CAS PubMed.
- R. J. Harvey and B. K. Yee, Nat. Rev. Drug Discovery, 2013, 12, 866–885 CrossRef CAS PubMed.
- B. Atkinson, S. Bell, M. De Vivo, L. Kowalski, S. Lechner, V. Ognyanov, C.-S. Tham, C. Tsai, J. Jia and D. Ashton, Mol. Pharmacol., 2001, 60, 1414–1420 CAS.
- A. Molander, H. H. LIDÖ, E. Löf, M. Ericson and B. Söderpalm, Alcohol Alcohol., 2007, 42, 11–18 CrossRef CAS PubMed.
- E. Pinard, A. Alanine, D. Alberati, M. Bender, E. Borroni, P. Bourdeaux, V. Brom, S. Burner, H. Fischer, D. Hainzl, R. Halm, N. Hauser, S. Jolidon, J. Lengyel, H.-P. Marty, T. Meyer, J.-L. Moreau, R. Mory, R. Narquizian, M. Nettekoven, R. D. Norcross, B. Puellmann, P. Schmid, S. Schmitt, H. Stalder, R. Wermuth, J. G. Wettstein and D. Zimmerli, J. Med. Chem., 2010, 53, 4603–4614 CrossRef CAS PubMed.
- W. P. Blackaby, R. T. Lewis, J. L. Thomson, A. S. R. Jennings, S. C. Goodacre, L. J. Street, A. M. MacLeod, A. Pike, S. Wood, S. Thomas, T. A. Brown, A. Smith, G. Pillai, S. Almond, M. R. Guscott, H. D. Burns, W. Eng, C. Ryan, J. Cook and T. G. Hamill, ACS Med. Chem. Lett., 2010, 1, 350–354 CrossRef CAS PubMed.
- D. Ouellet, S. Sutherland, T. Wang, P. Griffini and V. Murthy, Clin. Pharmacol. Ther., 2011, 90, 597–604 CrossRef CAS PubMed.
- D. Umbricht, D. Alberati, M. Martin-Facklam, E. Borroni, E. A. Youssef, M. Ostland, T. L. Wallace, F. Knoflach, E. Dorflinger and J. G. Wettstein, JAMA Psychiatry, 2014, 71, 637–646 CrossRef CAS PubMed.
- S. Jolidon, R. Narquizian, R. Norcross and E. Pinard, PCT Int. Appl. WO2007147770, 2007.
- P. Angell, K. J. Bordeau, Y. Chiang, N. Collar, D. M. Fink, H. Hemmerle, J. A. Hendrix, S. A. Jackson, J. G. Jurcak and T. Nieduzak, PCT Int. Appl. WO2002066446, 2002.
- A. K. Ghose, T. Herbertz, R. L. Hudkins, B. D. Dorsey and J. P. Mallamo, ACS Chem. Neurosci., 2011, 3, 50–68 CrossRef PubMed.
- Y. Chen, Y. Lan, X. Cao, X. Xu, J. Zhang, M. Yu, X. Liu, B.-F. Liu and G. Zhang, MedChemComm, 2015 10.1039/c4md00578c.
- P. A. Janssen, C. J. Niemegeers, F. Awouters, K. H. Schellekens, A. A. Megens and T. F. Meert, J. Pharmacol. Exp. Ther., 1988, 244, 685–693 CAS.
- S. Caccia, L. Pasina and A. Nobili, Drug Des., Dev. Ther., 2010, 4, 33–48 CrossRef CAS.
- M. Barcelo, E. Ravina, M. J. Varela, J. Brea, M. I. Loza and C. F. Masaguer, MedChemComm, 2011, 2, 1194–1200 RSC.
- Y. Guo, H. Zhang, X. Chen, W. Cai, J. Cheng, Y. Yang, G. Jin and X. Zhen, Schizophr. Res., 2009, 115, 41–49 CrossRef PubMed.
- P. R. Lee, D. L. Brady, R. A. Shapiro, D. M. Dorsa and J. I. Koenig, Neuropsychopharmacology, 2005, 30, 1883–1894 CrossRef CAS PubMed.
- K. Hashimoto, Y. Fujita, E. Shimizu and M. Iyo, Eur. J. Pharmacol., 2005, 519, 114–117 CrossRef CAS PubMed.
- K. R. Aubrey and R. J. Vandenberg, Br. J. Pharmacol., 2001, 134, 1429–1436 CrossRef CAS PubMed.
- J. Zhang, J. Huang, Z. Song, L. Guo, W. Cai, Y. Wang, X. Zhen and A. Zhang, Eur. J. Med. Chem., 2014, 85, 16–26 CrossRef CAS PubMed.
- H. Zhang, N. Ye, S. Zhou, L. Guo, L. Zheng, Z. Liu, B. Gao, X. Zhen and A. Zhang, J. Med. Chem., 2011, 54, 4324–4338 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of 10a–h, 13a–b, 16a–e, 18a–d and 21a–b. See DOI: 10.1039/c5ra04714e |
| This journal is © The Royal Society of Chemistry 2015 |