Template-free synthesis of porous boron nitride using a single source precursor†
Mahdi Maleki*a,
Ali Beitollahi*a and
Mohammadreza Shokouhimehrb
aCenter of Excellence for Ceramic Materials in Energy and Environment Applications, School of Metallurgy & Materials Engineering, Iran University of Science and Technology (IUST), Narmak, Tehran 16846, Iran. E-mail: malekim@metaleng.iust.ac.ir; beitolla@iust.ac.ir; Fax: +98 21 77459151; Tel: +98 21 77240480
bSchool of Chemical and Biological Engineering, College of Engineering, Seoul National University, Seoul 151-744, Republic of Korea
First published on 8th May 2015
Abstract
Porous boron nitride (BN) powder was prepared through a facile template-free synthesis route through the pyrolysis of controlled polymerized ammonia borane (AB) at 1300 °C. The prepared products were characterized using different techniques such as X-ray diffraction analysis, X-ray photoelectron spectroscopy, scanning transmission electron microscopy, transmission electron microscopy and N2 sorption analysis. We investigated the effect of AB polymerization temperature and time on the micropores formation and surface areas of the obtained products. Lower heating temperatures of AB before pyrolysis resulted in higher surface area and micropore volume. The highest surface area was obtained from isothermal heating of AB at 70 °C and 32 h before being subjected to pyrolysis at 1300 °C.
1. Introduction
Formation of micropores in organic or inorganic porous materials can be created by different methods. It already has been shown that removing organic templates such as polystyrene-b-polyethylene oxide (PS-b-PEO), Pluronic P123 and quaternary ammonium salts can generate micropores in porous materials.1–4 For instance, a mutually interpenetrating network of an inorganic precursor and hydrophilic PEO chains in PS-b-PEO led to micropores formation.1 Furthermore, small organic compounds such as quaternary alkyl ammonium salts have been used as structure directing agents in the synthesis of zeolite to make a pore network.2 Another route to obtain microporous structures is by applying molecular assembly of polymers in intrinsic microporous polymers.5 Synthesis of microporous polymers with rigid and contorted macromolecular structures which cannot fill space efficiently leaves voids in microporous polymers.5A micro/meso porous boron nitride (BN) nanostructure is one of the interesting ceramic materials that recently has been paid intensive attention due to its wide application potentials.6–8 To date, various procedures have been reported to fabricate nanoporous BN including hard and soft template methods for controlling pore size and shape of the BN products.9–13 However, template removal and frequent replications for hard template methods and complicated approaches for soft template procedures make them very impractical and inappropriate techniques to obtain nanoporous BN. Therefore, it is an important issue to develop economical and facile approaches for preparing porous BN nanostructures. Template-free methods have opened a new window in the facile synthesis of porous BN powders.14–16 In addition, a template-free procedure utilizing a single precursor for polymerization in special solvents has lent itself as an interesting route for the synthesis of some nanoporous materials such as aluminosilicates.17,18 T. T. Borek et al. reported the synthesis of microporous BN with single source and template-free procedure by polymerization of a BN polymeric precursor.19 However, in this work, using air sensitive and non-commercially available precursor could be considered as a disadvantage of such a preparation route.
Ammonia borane (AB), a white crystalline solid, was first prepared by Shore and Parry.20 AB is an appropriate precursor for the synthesis of BN structures due to its high contents of B and N and no oxygen and carbon. Although AB has polymerization ability, it has been frequently used for the synthesis of BN nanostructures through chemical vapor deposition methods and less attention has been paid for the BN production through the AB thermal decomposition.21–23 X. Wang et al. reported synthesis of BN nanosheets through a controlled activation of AB precursor followed by a precise pyrolysis program.24 They obtained crystalline BN nanosheets with a low specific surface area (130 m2 g−1). Herein, we report a facile synthesis of microporous/mesoporous BN nanostructures through a template-free method through controlled polymerization of a single source precursor (AB).
2. Experimental
2.1. Chemicals
Ammonia borane complex (97%) was purchased from Sigma-Aldrich Company.2.2. AB polymerization
0.2 g of AB powder was polymerized at 70, 80, 90 °C and 140 °C under N2 atmosphere in glass vial (Fig. S1†). Then, polymerized AB powders were pyrolyzed using a heating program of 1 °C min−1 ramping to 200 °C (for 2 h) followed by 1.4 °C min−1 ramping to 1150 °C under ammonia atmosphere and 1.4 °C min−1 to 1300 °C (for 5 min) under N2 atmosphere based on TGA/DSC results.The samples are named according to the abbreviations and applied reaction conditions with temperature and time. The polymerized and pyrolyzed samples begin with AB and BN, respectively. The detailed conditions of the prepared samples are shown in Table 1.
| Sample | Tpolymerization (°C) | tpolymerization (h) | Theat treatment (°C) | theat treatment (min) |
|---|---|---|---|---|
| AB.70.32h | 70 | 32 | — | — |
| AB.80.16h | 80 | 16 | — | — |
| AB.80.32h | 80 | 32 | — | — |
| AB.90.16h | 90 | 16 | — | — |
| AB.140.R | 140 | 2 | — | — |
| BN.13.70.32h | 70 | 32 | 1300 | 5 |
| BN.13.80.16h | 80 | 16 | 1300 | 5 |
| BN.13.80.32h | 80 | 32 | 1300 | 5 |
| BN.13.90.16h | 90 | 16 | 1300 | 5 |
| BN.13.140.R | 140 | 2 | 1300 | 5 |
2.3. Characterization
Powder X-ray diffractions (XRD) were obtained using an X'Pert Pro MPD diffractometer (Philips, CuKα, 1.54 Å). Fourier-transformed infrared (FT-IR) spectra were recorded on a PerkinElmer spectrophotometer between 500 and 4000 cm−1 using KBr pellets. X-ray photoelectron spectroscopy (XPS) measurements were carried out for the BN powders using an Al Kα source (Sigma probe, VG Scientifics). The carbonaceous C 1s line (284.8 eV) was used as the reference to calibrate the binding energies. Nitrogen physisorption was measured using a Belsorb system at −196 °C. The samples were degassed at 150 °C before measurements. The Brunauer–Emmett–Teller (BET) method was utilized to calculate the specific surface area (SBET) using the adsorption branch. The total pore volume (VP) was estimated from the adsorbed amount at relative pressure of 0.995. The Dubinin–Raduskevitch (DR) theory25 was used to estimate micropore volume. Transmission electron microscope (TEM) images were obtained using a JEOL EM-2010 microscope at an acceleration voltage of 200 kV. High resolution TEM (HRTEM) images were acquired using a JEOL JEM-3010 microscope at an acceleration voltage of 300 kV. Scanning transmission electron microscopy (STEM) images were obtained using a JEOL JEM-3000F. Thermogravimetric analysis was performed using a SDT Q600 V8.1 Build 99 instrument at heating rates of 10 °C min−1 under N2 atmosphere (flow rate of 100 ml min−1).3. Results and discussion
3.1. Polymerization of AB
To gain better understanding of the AB polymerization behaviour, the physical changes of AB powders during heating at 80, 90 and 140 °C were monitored by visual inspections (Fig. S2†). While continuous heating of AB to 140 °C, the melting occurred at 108 °C which is consistent with the TGA/DSC results (Fig. S3†). The precursor melting accompanied with hydrogen evolution led to foaming and swelling. The volume of AB increased approximately more than 10 times of the initial volume.Similar voluminous swelling properties have been reported by other research groups during the AB heating.26,27 After the completion of AB melting, the swelling stopped and the volume of the foam powder decreased slightly. At the isothermal heating of AB at 90 °C (AB.90.16h), melting and swelling occurred after 2 h heating. As the hydrogen evolution is an exothermic reaction,28 the released heat could provide the required energy for the AB melting before reaching its melting point. However, the volume of swelled powders at 90 °C was less than the rapid heating. At the isothermal heating of AB at 70 and 80 °C, there was no observable change in the physical properties of the heated AB powders even after 32 h.
Further characterizations such as XRD and FT-IR were used to study the AB decomposition products under rapid and isothermal heating conditions.
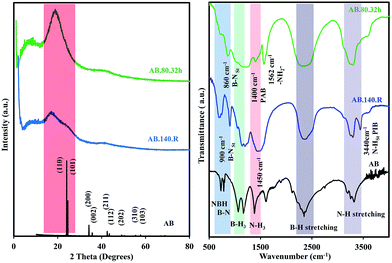
The XRD patterns of AB.80.32h and AB.140.R compared to AB are shown in Fig. 1. The XRD spectrum of AB revealed peaks at 24, 24.5, 34.2, 35.7, 42.6, 43.5, 49.2, 55.4 and 57.7 degrees. The observed peaks are attributed to (110), (101), (200), (002), (211), (112), (202), (310) and (103) reflections in the tetragonal structure of AB (JCPDS Card no. 13-0292). As can be seen in Fig. 1, after polymerization of AB at 80 °C (AB.80.32h), all AB peaks disappeared and a new shoulder was formed between 19–21 degrees. The appearance of such a shoulder could be the sign of polyaminoborane (PAB) formation as a main product of AB decomposition.26 The XRD pattern of AB.140.R also revealed an amorphous structure with lower reflections compared to the AB.80.32h sample. These distinctions are due to different decomposition products from different decomposition routes and temperature.
The FT-IR spectra comparison of AB and AB.140.R revealed that the N–H bonds have shifted into a higher wavenumber for AB.140.R (Fig. 1). The appearance of a peak at 3435 cm−1 demonstrated the presence of the double bond between nitrogen and hydrogen. This could be attributed to polyiminoborane (PIB) formation. Furthermore, the peak of 1450 cm−1 in AB.140.R sample is resulted from the net-shaped compounds similar to polyborazines.29 In addition, the presence of the B–H bond in a higher wavenumber could be another reason for the formation of a PIB-like product during the rapid heating.30 In the sample AB.80.32h, the N–H peaks were moved to lower wavenumbers and become broader relative to AB peaks. The presence of a peak at 1562 cm−1 could be due to –NH2– oligomers of PAB.31 The peak of 1400 cm−1 obtained in the AB.80.32h sample is assigned to PAB.30 Therefore, the most probable product is PAB in the isothermal heating process while in the rapid heating procedure the net-shaped product were formed in addition to the PAB product.
3.2. BN formation
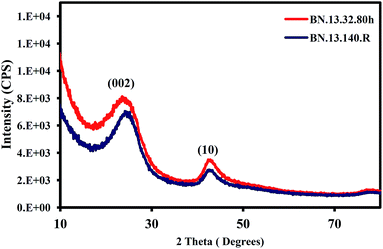
Fig. 2 demonstrates the XRD patterns of BN.13.140.R and BN.13.80.32h samples revealing the existence of two broad peaks around 20–28 and 40–45 degrees. These peaks are assigned to (002) and (10) in t-BN structure, respectively.10As can be noticed from Fig. 2, slightly higher crystallinity could be obtained for the sample already subjected to polymerization at 140 °C (BN.13.140.R sample). Different polymer products resulted from the AB decomposition may cause these changes. It has been reported that the BN precursors with cyclic-shaped structures have memory effect in the formation of highly crystalline BN.30 Moreover, the presence of highly disordered structures due to presence of micropores could be also possibly responsible for this effect and this will be considered later.
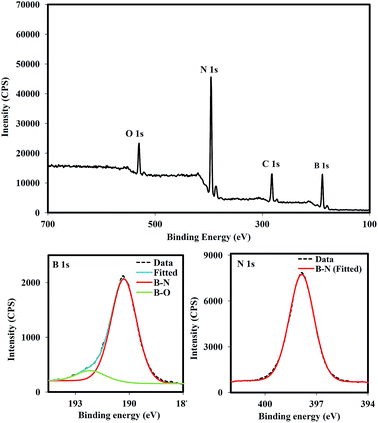
To further investigate the chemical states of the prepared powders, XPS analysis was used for the sample BN.13.80.32h. Fig. 3 shows the survey scan, B 1s and N 1s spectra of the prepared powder. The full survey scan spectrum revealed the presence of boron, carbon, nitrogen and oxygen in the sample. Since the AB does not have oxygen and carbon, the appearance of such elements in the spectrum could be due to surface contaminations of the pyrolyzed sample during the exposure to air before the analysis. Further reason for presence of the element oxygen could be attributed to oxidation of polymerized AB during its transfer to the furnace. However, as PAB is stable in the air,31 this reason is not most likely. The B 1s spectrum was fitted with two curves using a Gaussian profile. The main one with a binding energy peak at ∼190.29 eV is assigned to B–N bonds and the other one at ∼191.81 eV is attributed to a B–O bond.15,32 Owing to higher electronegativity of oxygen than nitrogen, this bonding configuration led to the shift of the peak to higher binding energies. The N 1s peak revealed a symmetrical Gaussian line shape which peaked at 398.3 eV. Both the B 1s and the N 1s spectra indicate that the configuration for B and N atoms is the B–N bond, implying BN formation.15
3.3. Surface area of the prepared BN powders
N2 sorption isotherms of the pyrolyzed samples are depicted in Fig. 4. The adsorption isotherm of BN.13.140.R and BN.13.90.16h samples could be categorized as a type II isotherm that is a characteristic of macroporous materials. The polymerized samples at 70 and 80 °C have a type I adsorption isotherm approximately that is assigned to microporous powders. The appearance of hysteresis is due to presence of mesopores in the synthesized samples. Decreasing the polymerization temperature before pyrolysis led to a rise of N2 uptake at low relative pressure below 0.05. This is the sign of an increased level of micropores by the lower polymerization temperature. Moreover, a higher N2 uptake at low relative pressure was obtained for the longer polymerization time (32 h) compared to 16 h at 80 °C. These observations are consistent with derived data from isotherms in Table 2. Decreasing the polymerization temperature enhanced the SBET and Vmicro. The highest SBET (560 m2 g−1) and Vmicro (0.222 cm3 g−1) were obtained at the lowest polymerization temperature of 70 °C (BN.13.70.32h). This high surface area is comparable with some reports related to synthesis of porous BN through hard template methods.33,34 High surface area BN powders (437–712 m2 g−1) were prepared using a single source of BN polymeric precursors.35 However, synthesis of such air sensitive polymeric precursors (borazine compounds) that are not commercially available is a main constraint for such a preparation approach.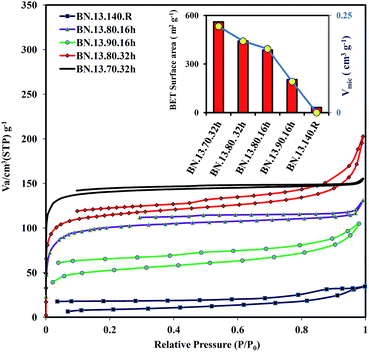 | ||
| Fig. 4 Nitrogen adsorption–desorption isotherm of the pyrolyzed samples. The inset is the summary of BET surface areas and Vmicro for the obtained samples. | ||
| Sample | SBET | Vtotal | Vmicro |
|---|---|---|---|
| BN.13.70.32h | 560 | 0.245 | 0.222 |
| BN.13.80.16h | 386 | 0.2 | 0.164 |
| BN.13.80.32h | 442 | 0.31 | 0.184 |
| BN.13.90.16h | 204 | 0.162 | 0.08 |
| BN.13.140.R | 31.6 | 0.005 | — |
3.4. Morphology of the obtained BN powders
TEM images of the BN.13.90.16h, BN.13.80.32h and BN.13.70.32h samples are shown in Fig. 5. The TEM image of the BN.13.90.16h sample does not reveal the existence of a noticeable level of micropores and this is confirmed by BET results as well (Fig. 5a and b). However, a STEM image of this sample in Fig. 5c demonstrated the presence of mesopores in the powders. Formation of these mesopores could be due to the second step of hydrogen evolution. TEM images of the BN.13.80.32h sample in Fig. 5d and e confirm the formation of mesoporous and microporous structures. However, both mesopores and micropores in these samples have disordered morphologies. The sample of BN.13.70.32h revealed sheet-like morphology (Fig. 5f) similar to the result reported by the X. Wang group.24,36 The formation of sheet-like morphology was related to PAB bubbles. The TEM image of the BN.13.70.32h sample at a higher magnification also revealed the presence of large pores and wormlike micropores (Fig. 5g and h). Stack atomic layers of BN could be detected in micropores walls as shown in Fig. 5i. Such a very small crystalline domain could be responsible for appearance of the observed broad XRD reflections at lower degrees compared to crystalline h-BN.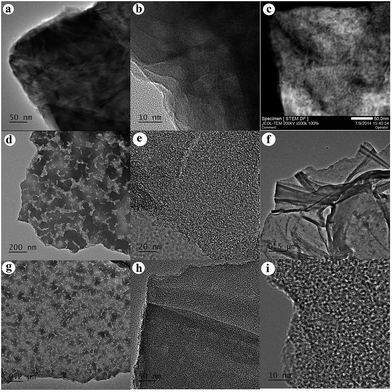 | ||
| Fig. 5 (a) TEM, (b) HRTEM and (c) STEM images of sample BN.13.90.16h. (d) TEM and (e) HRTEM images of sample BN.13.80.32h. (f–h) TEM and (i) HRTEM images of sample BN.13.70.32h. | ||
3.5. Micropores formation
P. R. Malenfant et al. reported that using a BN precursor with polymerization ability could lead to formation of microporous powders.9 D. M. Smith et al. synthesized a porous BN powder with a high surface area through vacuum pyrolysis of (poly(4,6-borazinylamine)).19,35 In addition, pyrolysis of supercritical dried gel containing a poly(borazinylamine) and tetrahydrofuran led to formation of BN aerogel.37 The mechanism of AB decomposition has been extensively explored by other research groups. The overall BN formation from AB decomposition is shown in Fig. 6. As can be seen, the first step of AB decomposition is formation of PAB followed by PIB and BN. This critical step between AB and PAB was considered properly by W. J. Shaw's group.38 X. Wang also reported this step has a critical role in the formation of BN nanosheets.36 A. C. Stowe et al. stated that the decomposition of AB started with disruption of dehydrogen bonding and formation of a mobile phase (Fig. 6).39 Diammoniate of diborane (DADB) is nucleated from an AB mobile phase. After that, DADB reacts with remaining AB to form linear chains. With more time, linear chains then connect together.39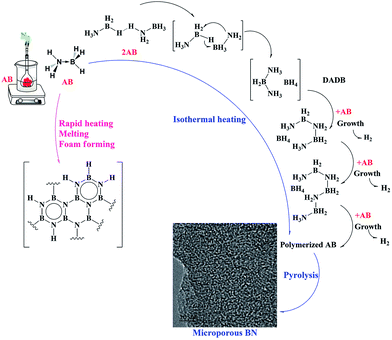 | ||
| Fig. 6 Schematic presentation of thermal decomposition of AB.39,40 | ||
As mentioned earlier in this work, the most probable product in isothermal heating could be a linear polymer of PAB while with rapid heating cyclic-like products could possibly have been formed.
With long isothermal heating, the growth and connection of this linear polymeric chain leaves some space between the long linear chains. These inter-space voids probably act as a backbone of micropores. Further, with rapid heating, the linear chain of AB does not have enough time for further polymerization and melting disrupts the probable micropore backbones. Of course, further detailed characterization is required to support this assumption.
4. Conclusion
In summary, pyrolysis of controlled polymerized AB at 1300 °C led to formation of high surface area microporous BN powder. Polymerization of AB before pyrolysis has a critical role in micropore formation. Lower temperature heating of AB before pyrolysis resulted in a higher surface area and pore volume and micropore volume. The highest surface area was obtained by heating AB at 70 °C and 32 h before pyrolysis at 1300 °C.Notes and references
- B. Smarsly, G. Xomeritakis, K. Yu, N. Liu, H. Fan, R. A. Assink, C. A. Drewien, W. Ruland and C. J. Brinker, Langmuir, 2003, 19, 729 CrossRef.
- R. Kore and R. Srivastava, RSC Adv., 2012, 2, 10072 RSC.
- A. Galarneau, H. Cambon, F. Di Renzo, R. Ryoo, M. Choi and F. Fajula, New J. Chem., 2003, 27, 73 RSC.
- C. M. Yang, B. Zibrowius, W. Schmidt and F. Schüth, Chem. Mater., 2004, 16, 2918 CrossRef CAS.
- J. Vile, M. Carta, C. G. Bezzu and N. B. McKeown, Polym. Chem., 2011, 2, 2257 RSC.
- Q. Weng, B. Wang, X. Wang, N. Hanagata, X. Li, D. Liu, X. Wang, X. Jiang, Y. Bando and D. Golberg, ACS Nano, 2014, 8, 6123 CrossRef CAS PubMed.
- Q. Weng, X. Wang, C. Zhi, Y. Bando and D. Golberg, ACS Nano, 2013, 7, 1558 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- P. R. Malenfant, J. Wan, S. T. Taylor and M. Manoharan, Nat. Nanotechnol., 2007, 2, 43 CrossRef CAS PubMed.
- P. Dibandjo, L. Bois, F. Chassagneux, D. Cornu, J.-M. Letoffe, B. Toury, F. Babonneau and P. Miele, Adv. Mater., 2005, 7, 571 CrossRef PubMed.
- M. Maleki, A. Beitollahi, J. Javadpour and N. Yahya, Ceram. Int., 2015, 41, 3806 CrossRef CAS PubMed.
- M. Maleki, A. Beitollahi, J. Lee, M. Shokouhimehr, J. Javadpour, E. J. Park, J. Chun and J. Hwang, RSC Adv., 2015, 5, 6528 RSC.
- M. Maleki, A. Beitollahi and M. Shokouhimehr, Eur. J. Inorg. Chem., 2015, 2015, 2478 CrossRef CAS PubMed.
- Q. Weng, X. Wang, Y. Bando and D. Golberg, Adv. Energy Mater., 2014, 4, 1301525 Search PubMed.
- D. Liu, W. Lei, S. Qin and Y. Chen, Sci. Rep., 2014, 4, 4453 Search PubMed.
- H. Zhao, X. Song and H. Zeng, NPG Asia Mater., 2015, 7, e168 CrossRef CAS PubMed.
- A. Lemaire and B. L. Su, Microporous Mesoporous Mater., 2011, 142, 70 CrossRef CAS PubMed.
- A. Lemaire and B.-L. Su, Langmuir, 2010, 26, 17603 CrossRef CAS PubMed.
- T. T. Borek, W. Ackerman, D. W. Hua, R. T. Paine and D. M. Smith, Langmuir, 1991, 7, 2844 CrossRef CAS.
- S. G. Shore and R. W. Parry, J. Am. Chem. Soc., 1955, 77, 6084 CrossRef CAS.
- A. Pakdel, C. Zhi, Y. Bando, T. Nakayama and D. Golberg, ACS Nano, 2011, 5, 6507 CrossRef CAS PubMed.
- J. Yu, L. Qin, Y. Hao, S. Kuang, X. Bai, Y.-M. Chong, W. Zhang and E. Wang, ACS Nano, 2010, 4, 414 CrossRef CAS PubMed.
- K. K. Kim, A. Hsu, X. Jia, S. M. Kim, Y. Shi, M. Hofmann, D. Nezich, J. F. Rodriguez-Nieva, M. Dresselhaus, T. Palacios and J. Kong, Nano Lett., 2011, 12, 161 CrossRef PubMed.
- X. Wang, C. Zhi, L. Li, H. Zeng, C. Li, M. Mitome, D. Golberg and Y. Bando, Adv. Mater., 2011, 23, 4072 CrossRef CAS PubMed.
- N. D. Hutson and R. T. Yang, Adsorption, 1997, 3, 189U CrossRef.
- U. B. Demirci, S. Bernard, R. Chiriac, F. Toche and P. Miele, J. Power Sources, 2011, 196, 279 CrossRef CAS PubMed.
- R. Chiriac, F. Toche, U. B. Demirci, O. Krol and P. Miele, Int. J. Hydrogen Energy, 2011, 36, 12955 CrossRef CAS PubMed.
- Y. Zhao, J. Zhang, D. L. Akins and J. W. Lee, Ind. Eng. Chem. Res., 2011, 50, 10024 CrossRef CAS.
- M. R. Weismiller, S. Q. Wang, A. Chowdhury, S. T. Thynell and R. A. Yetter, Thermochim. Acta, 2013, 551, 110 CrossRef CAS PubMed.
- D. P. Kim, K. T. Moon, J. G. Kho, J. Economy, C. Gervais and F. Babonneau, Polym. Adv. Technol., 1999, 10, 702 CrossRef CAS.
- R. Komm, R. A. Geanangel and R. Liepins, Inorg. Chem., 1983, 22, 1684 CrossRef CAS.
- C. Guimon, D. Gonbeau, G. Pfister-Guillouzo, O. Dugne, A. Guette, R. Naslain and M. Lahaye, Surf. Interface Anal., 1990, 16, 440 CrossRef CAS PubMed.
- P. Dibandjo, F. Chassagneux, L. Bois, C. Sigala and P. Miele, J. Mater. Chem., 2005, 15, 1917 RSC.
- S. Schlienger, J. Alauzun, F. Michaux, L. Vidal, J. Parmentier, C. Gervais, F. Babonneau, S. Bernard, P. Miele and J. B. Parra, Chem. Mater., 2011, 24, 88 CrossRef.
- J. F. Janik, W. C. Ackerman, R. T. Paine, D. W. Hua, A. Maskara and D. M. Smith, Langmuir, 1994, 10, 514 CrossRef CAS.
- X. Wang, A. Pakdel, C. Zhi, K. Watanabe, T. Sekiguchi, D. Golberg and Y. Bando, J. Phys.: Condens. Matter, 2012, 24, 314205 CrossRef PubMed.
- D. A. Lindquist, T. T. Borek, S. J. Kramer, C. K. Narula, G. Johnston, R. Schaeffer, D. M. Smith and R. T. Paine, J. Am. Ceram. Soc., 1990, 73, 757 CrossRef CAS PubMed.
- W. J. Shaw, M. Bowden, A. Karkamkar, C. J. Howard, D. J. Heldebrant, N. J. Hess, J. C. Linehan and T. Autrey, Energy Environ. Sci., 2010, 3, 796 CAS.
- A. C. Stowe, W. J. Shaw, J. C. Linehan, B. Schmid and T. Autrey, Phys. Chem. Chem. Phys., 2007, 9, 1831 RSC.
- S. Bernard and P. Miele, Mater. Today, 2014, 17, 443 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04636j |
| This journal is © The Royal Society of Chemistry 2015 |