ESR/UV-Vis-NIR spectroelectrochemical study and electrochromic contrast enhancement of a polythiophene derivative bearing a pendant viologen†
Abstract
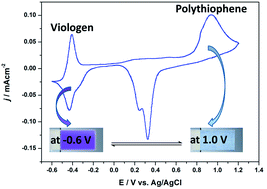
The importance of viologens in the field of electrochromic materials is well recognized due to their intensely colored radical cation formation. In this study, 1-[6-[(4-methyl-3-thienyl)oxy]hexyl]-4,4′-bipyridium hexafluorophosphate (Th-V) was synthesized and electropolymerized in a solvent mixture comprising water and acetonitrile (v/v; 50 : 50) with 0.1 M lithium perchlorate (LiClO4) as an electrolyte salt, yielding a viologen bearing polythiophene (PTh-V) film on an electrode surface. The resulting polymer shows electrochemical activity from both the redox active viologen and the conjugated polythiophene moieties. The redox behavior of the polymer was studied by multi in situ spectroelectrochemical technique by means of simultaneous recording of electron spin resonance and UV-Vis-near infrared (ESR/UV-Vis-NIR) spectra. The results indicate that only polaron charge carriers are created during both n- and p-doping of the PTh-V film. The polymer film shows enhanced electrochromic contrast due to the introduction of a pendant viologen group into the thiophene unit. The film switched reversibly between dark violet (at −0.6 V) and almost transparent (at 1.0 V) showing good optical contrast with a coloration efficiency of ca. 305 cm2 C−1 at 610 nm. The switching transmittance kinetics demonstrate fast response times to attain a bleached state and excellent operational stability with repeatable voltage switching between colored/bleached states for 1000 cycles. The polythiophene backbone was found to strengthen the thermal stability of the conjugated PTh-V redox polymer. The excellent optical contrast with sharp color changes and high color efficiency combined with adequate thermal behavior suggests the potential of PTh-V in the electrochromic device (ECD) application.

 Please wait while we load your content...
Please wait while we load your content...