Four highly efficient cuprous complexes and their applications in solution-processed organic light-emitting diodes†
Qing Zhangabc,
Xu-Lin Chenab,
Jun Chenc,
Xiao-Yuan Wuab,
Rongmin Yuab and
Can-Zhong Lu*ab
aKey Laboratory of Design and Assembly of Functional Nanostructures, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: czlu@fjirsm.ac.cn; Fax: +86-591-8370-5794
bFujian Provincial Key Laboratory of Nanomaterials, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
cGraduate University of Chinese Academy of Sciences, Beijing 100049, China
First published on 8th April 2015
Abstract
Four mononuclear cationic Cu(I) complexes featuring functional C-linked pyrazolyl pyridine diimine ligands have been synthesized and characterized. Under UV 365 nm at room temperature, these complexes emit similar orange light in solution and green light in thin film, which could be attributed to the limited charge and steric influence of these methyl or trifluoromethyl substituted phenyl appendages at the pyrazol ring. Moreover, multilayer organic light-emitting diodes (OLEDs) based on these Cu(I) complexes had different performance efficiencies. The device with complex 1 as the light emitting material had the highest current efficiency of 17.8 cd A−1 and an external quantum efficiency (EQE) of 6.4%, while the device with complex 2 which shows the highest photoluminescence quantum yields gave the poorest device efficiency with a current efficiency of 13 cd A−1 and an EQE of 4.7%.
Introduction
Since the pioneering work of Tang et al.,1 multilayered organic light-emitting diodes (OLEDs) have attracted considerable attention.2–4 In the emitting layers of OLEDs, dopants are one of the most important components because they are keys to the electrochemical stability, decay time, color, efficiency, etc. of the devices.5 Up to the present, there are two kinds of dopants for highly efficient OLEDs which can surpass 25% of the theoretical intrinsic upper limit of conventional fluorescent emitters. One is the triplet harvesting phosphorescent materials, including third-row noble Os(II),6–8 Ir(III)9–13 and Pt(II)14,15 complexes.16 Another is thermally activated delayed fluorescence17–19 (TADF) materials containing pure organic skeleton compounds20–22 and specific cuprous complexes23–25 which are low-cost and environmentally benign.In order to have highly efficient cuprous complexes, “small energy gap between T1 and S1” and “large energy gap between T1 and S0” are required. The former determines the level of thermally up-conversion from T1 to S1.26,27 And the later probably hinder the thermal population radiationless deactivation.28 For a Cu(I) complex with distorted tetrahedral coordination geometry, it undergoes a typical flattening distortion process to offer a lower T1 state when it is excited.29,30 Due to this flattening geometry, the Cu(I) ion becomes easier to be accessed by solvent and oxygen molecular, which, subsequently, accelerate the emission quenching.31 The introduction of the large steric ligand bis[2-(dipenylphosphino)phenyl]ether (POP) could effectively block the flattening process, and then increase the efficiency of the photoluminescence.5,32–34 In these heteroleptical Cu(NN)(POP)+ complexes, the HOMOs have predominant contribution from the metal d orbital and the POP ligand, and the LUMOs are on the diimine ligands, indicating an emission from metal and ligand to ligand charge transfer ((M + L)LCT)) excited state.35,36 The HOMOs of these complexes were lowered down by the electron deficient POP.33 Meanwhile, their LUMOs and T1 levels can be further elevated by the introduction of electron-rich and bulky steric hindrance diimine ligands which has already led to highly efficient photo- and electro-luminescence of Cu(I) complexes.37,38
Although neutral Cu(I) complexes without small counterions achieve higher device performance than those with counterions,39–44 these complexes containing N−-ligands and halogen counterions have much lower stability45–47 or are more difficult to adjust the emission spanning the whole visible region.48–52 Cationic emissive Cu(I) complexes are more stable and their emission would be easily adjusted.27,53–55 Moreover, these complexes with diimine and POP ligands had been successfully fabricated into devices and excellent external quantum efficiency (EQE) up to 16% through the cost-effective solution spin-coating method had been realized.56
In this study, four novel cationic cuprous complexes based on functional 3-C-linked pyrazolyl pyridine diimine ligands were synthesized and characterized. Significant different influences of functional groups for these complexes both on the photo- and electro-luminescence as compared to our previous reported blue-emitting cationic Cu(I) complexes37 have been observed. For the complexes with functional N-linked 2-pyrazolyl pyridine diimine ligands, the substituent group largely influence their emission properties both on photo- and electro-luminescence, meanwhile, the trifluoromethyl substituted compounds showed the best device efficiency. The complexes in this report had similar photo-luminescence behaviours in PMMA and in dichloromethane, respectively, which suggest that the influences of the substituent groups are negligible. Moreover, complex 1 without trifluoromethyl and methyl group had shown the best device efficiency with the highest current efficiency of 17.8 cd A−1 and EQE of 6.4% with the configuration: ITO/PEDOT:PSS(40 nm)/10 wt% of 1 (or 2, 3, 4):host(30 nm)/DPEPO(50 nm)/LiF(0.7 nm)/Al(100 nm). While, the device based on 2 gave the poorest device efficiency with current efficiency of 13 cd A−1 and EQE of 4.7%.
Results and discussion
Synthesis and characterization
According to previous report,37 cuprous complexes [Cu(phpzpy)(POP)]BF4 (1), [Cu(2-mphpzpy)(POP)]BF4 (2), [Cu(2-tfphpzpy)(POP)]BF4 (3), [Cu(4-tfphpzpy)(POP)]BF4 (4) (Fig. 1) were prepared from [Cu(CH3CN)4]BF4 with one equivalent of POP and corresponding diimine ligand in dichloromethane. The as-made solids were purified and isolated through crystallization by layering diethyl ether on the surface of the dichloromethane solvent of these complexes. The obtained complexes were characterized with 1H NMR, 31P NMR, thermal analysis, elemental analysis and Single-crystal X-ray diffraction analysis.The crystals for X-ray analysis were recrystallized in the dichloromethane/diethyl ether for 1 and in the ethanol for 2–4 as shown in Fig. 2. Selected bond distances and bond angles were listed in Table S2.† Similar highly distorted tetrahedral geometries were observed for these complexes with N–Cu–N and P–Cu–P angles within the range of 78.46–79.34° and 109.05–114.85°, respectively. The Cu–N2 bond length was shorter than that of Cu–N3, suggesting a stronger coordination between copper ion and pyrazol unit than that between copper ion and pyridine ring.
 | ||
| Fig. 2 ORTEP diagrams of 1–4 with thermal ellipsoids at 30% probability level. Solvent molecules, the anion, and H atoms were omitted for clarity. | ||
Photophysical properties
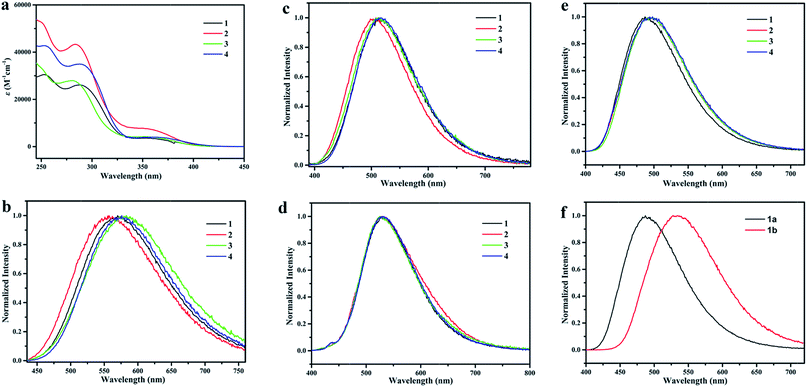
Photophysical data of these complexes in the degassed CH2Cl2, PMMA and crystal powder were listed in Table 1. Their corresponding UV-vis absorption and emission spectra were shown in Fig. 3a. According to the previous studies,38,57 the intense absorption bands below 300 nm belong to spin-allowed π–π* ligand-centered (LC) transitions of diimine ligand and POP ligand, and the tailed peak from 330 nm to 420 nm is attributed to the MLCT transition from the Cu(I) ion to diimine ligand. The onset wavelengths of absorption spectra which used to estimate the energy gap (ΔEg) between HOMO and LUMO were consistent with the peak of excitation spectra (Fig. S3†). Similar ΔEg values of these complexes were obtained for 1 (3.04 eV), 2 (3.08 eV), 3 (3.04 eV) and 4 (3.00 eV). Therefore, parallel emission spectra of these four complexes probably could be observed.| Solution (in degassed CH2Cl2) | PMMA films | Powder | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) (ε × 10−3 M−1 cm−1) | λema (nm) | τb (μs) | λema (nm) | τb (μs) | Φc (%) | λema (nm) | τb (μs) | Φc (%) | |
| a Emission maximum.b Emission decay time (monoexponential fit, error ± 5%).c Photoluminescence quantum yield (error ± 10%) at 298 K. | |||||||||
| 1 | 253(31), 288(26), 357(3.4) | 570 | 5.7 | 513 | 38.1 | 53 | 487 | 41 | 72 |
| 2 | 245(54), 284(43), 349(7.8) | 556 | 4.2 | 506 | 38.2 | 47 | 494 | 61 | 91 |
| 3 | 245(35), 280(28), 354(4.1) | 580 | 4.8 | 511 | 34.2 | 54 | 494 | 43 | 77 |
| 4 | 253(43), 287(35), 361(4.1) | 576 | 5.2 | 515 | 38.8 | 46 | 498 | 49 | 70 |
In the CH2Cl2, all of these four complexes displayed bright yellow-orange emission under UV 365 nm (Fig. 3b). Quite similar emission wavelength and decay time of 3 and 4 suggest that the position of the steric trifluoromethyl group has limited influence on their emitting light. Additionally, the emission spectra of 3 and 4 had several nanometers of red shift, and 2 with methyl group had 14 nm of blue shift compared with that of 1. These results suggested that the emission spectra of these four complexes were mainly controlled by the charge character instead of the position of substituent group. Furthermore, 1 had the longest decay time among them, probably because it had not vibration of the appended methyl and trifluoromethyl groups as other complexes did. Certainly, complex 2 had the shortest emission life due to its additional C–H vibration of methyl unit.58
In comparison with these complexes in the CH2Cl2, their emission in the PMMA films had blue shift of at least 50 nm at room temperature (Fig. 3c), their decay times were increased nearly by six times and their quantum yields were significantly improved. The changes for their emission behaviours are caused by the suppressed geometry distortion and non-radiative quenching process in the excited state with the increasing in rigidity of the matrix.52,53 Meanwhile, the emission spectra for all of these four cuprous complexes at 77 K are red shift (Fig. 3d) which caused by the changes of the light emitting states (from the singlet to the triplet state). The changes suggest that the triplet state (T1) could be thermally activated to the singlet state (S1) for these complexes at room temperature. The hypothesis is consistent with the small energy gaps for these complexes estimated from the onsets of their emission peaks in PMMA, 0.16 eV for 1, 0.24 eV for 2, 0.16 eV for 3 and 0.17 eV for 4 which is small enough for thermally up-conversion.
Highly photoluminescence quantum yields (up to 90%) of these four cuprous complexes in the solid state prepared in the ethanol suggested that the effects of energy transferring between adjacent emitter molecules were not significant. Similar luminescence performances were also observed except slightly blue shift emission spectrum of 1 and increase in decay time and quantum yield of 2. However, because copper is internal heavy metal,59 its MLCT exited state was largely influenced by the morphology even in the solid state.36 As shown in Fig. 3f, complex 1 had 45 nm of emission spectral shift from blue-green of the crystal state prepared in ethanol to green of the solid prepared in CH2Cl2/Et2O, and the emission peak of later state even had 20 nm of red shift compared with that of 1 in PMMA.
In order to understand the nature of the emitting state of these complexes, 1 was selected as a representative, its emission spectra in the solid state prepared in CH2Cl2/Et2O was measured at 77 K and 298 K (Fig. 4a). The results showed that 1 had two inter-convertible exited states with 28 nm of emission spectral shift, which was confirmed by the decay time of 1 at varied temperatures from 77 K to 298 K as listed in Table S3.† These data were tested with the following TADF equations.17–19,27
Herein, kB and T are the Boltzmann constant and the absolute temperature, respectively. τ(S1) and τ(T1) are the individual decay times of the lowest singlet and triplet excited state, and ΔEST is the energy gap between these two states. The fitted values of τ(S1), τ(T1) and ΔEST are 300 ns, 287 μs and 0.10 eV, respectively. At 77 K, the τobs is approximately assigned to pure T1 state (Fig. 4b). The ΔEST value agrees very well with the estimated energy difference (0.12 eV) between onset of emission spectra at 77 K and 298 K. The small ΔEST is essential to facilitate thermal up-conversion from T1 to S1 at room temperature. The results showed that these data fitted very well with the TADF equation, suggesting these cuprous complexes can largely harvest the energy of T1 state through thermally up-conversion and are promising alternatives for highly efficient OLEDs.
Electroluminescence performances
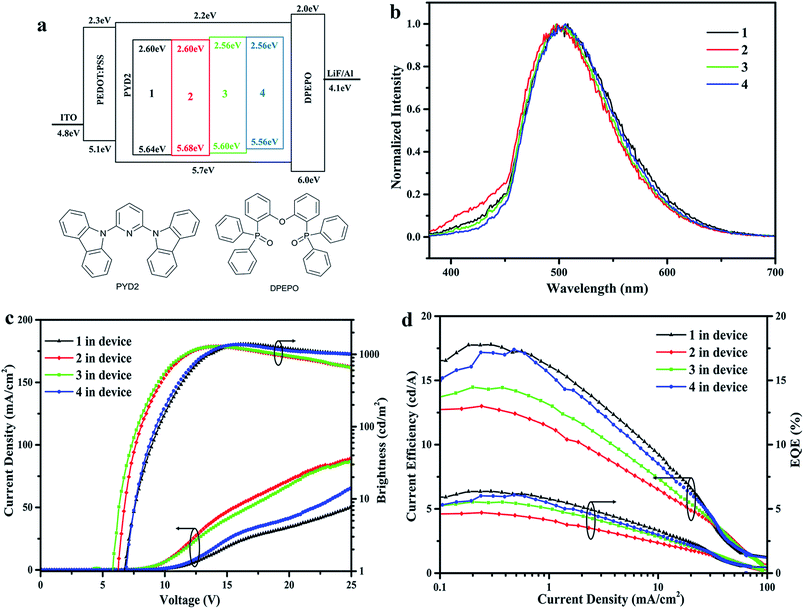
The tiled cuprous complexes were selected for solution processed OLED fabrication with the configuration: ITO/PEDOT:PSS (40 nm)/10 wt% of 1 (or 2, 3, 4):PYD2 (2,6-bis(N-carbazolyl)pyridine, 30 nm)/DPEPO (bis[2-(di-(phenyl)phosphino)-phenyl]ether oxide, 50 nm)/LiF (0.7 nm)/Al (100 nm) to evaluate their electroluminescence performances. In this configuration, PYD2 and DPEPO acted as hole-transporting and electron-transporting materials, respectively. The HOMO/LUMO levels of these complexes were determined by oxidation potential and their onsets of absorption spectra (5.64/2.6 eV for 1), (5.68/2.6 eV for 2), (5.6/2.56 eV for 3) and (5.56/2.56 eV for 4). The corresponding HOMO/LUMO levels of other materials used in the device were based on previous reports.37 The structures of organic materials and the energy levels of device layers were shown in Fig. 5a. The functional groups on 3-C-linked pyrazolyl pyridine diimine ligands had different influence on electroluminescence compared with substituent groups of N-linked pyrazolyl pyridine diimine ligands. As shown in Fig. 5b–d and Table 2, these four cuprous complexes had similar green electroluminescent (EL) spectra with slightly blue shift emission spectra compared with their photoluminescence in PMMA, which might be due to the different rigidity of the matrix. Additionally, devices based on 2 and 3 exhibited relatively poor EL performances with turn-on voltage (Von) of 6.3 eV and 5.9 eV, maximum current efficiency (CEmax) of 13.0 cd A−1 and 14.5 cd A−1, EQE of 4.71% and 5.54%, maximum brightness (Bmax) of 1286 cd m−2 and 1281 cd m−2, respectively. Device with Von of 6.9 eV, CEmax of 17.8 cd A−1, EQE of 6.37%, Bmax of 1378 cd m−2 had been realized based on 1, and a slightly poorer efficiency was observed for 4. These results showed that the substituent groups on appendage phenyl are not in favour of the highly efficient device performance for this series of complexes, and the reasons for this are still needed to be verified.| Dopant | CEmax (cd A−1) | EQEmax (%) | Von (eV) | Bmax (cd m−2) | λmax (nm) | CIE1931 (x, y) |
|---|---|---|---|---|---|---|
| 1 | 17.8 | 6.4 | 6.9 | 1378 | 508 | (0.22,0.45) |
| 2 | 13.0 | 4.7 | 6.3 | 1286 | 498 | (0.22,0.44) |
| 3 | 14.5 | 5.5 | 5.9 | 1281 | 498 | (0.21,0.41) |
| 4 | 17.4 | 6.1 | 6.9 | 1366 | 506 | (0.23,0.46) |
Conclusions
In this study, four novel highly emissive cationic cuprous complexes based on functional 3-C-linked pyrazolyl pyridine diimine ligands had been synthesized and characterized. It was found that the influence of substituent methyl and trifluoromethyl on photoluminescence was very limited at room temperature. These complexes all had similar photoluminescence behaviours both in the degassed CH2Cl2 and PMMA. However, different performance efficiencies of these complexes were observed in the solution processed OLEDs. Results of PL and EL had also demonstrated that cuprous complex 1 with substituent-free diimine ligand should be a more effective dopant material of OLEDs.Experimental
General
All experiments were performed under N2 atmosphere with standard Schlenk techniques unless specified. Solvents were freshly distilled over appropriate drying reagents. Other materials were purchased and used as received. [Cu(CH3CN)4]BF4 was prepared according to the literature procedure.60Characterization
Samples for NMR, elemental analyses and thermogravimetric analysis (TGA) were dried at 90 °C for 30 min. 1H NMR and 31P NMR spectra were recorded on a Bruker Avance III 400 MHz NMR spectrometer. Elemental analyses (C, H, N) were determined with an Elementar Vario EL III elemental analyzer. TGA were done on a NETZSCH STA 449C Jupiter thermogravimetric analyzer with flowing nitrogen and Al2O3 crucible at a heating rate of 10 K min−1 (Fig. S2†). Cyclic voltammetry (CV) was performed in a gastight single-compartment three-electrode cell with a BAS Epsilon electrochemical analyzer at room temperature. A glassy carbon disk and a platinum wire were selected as the working and auxiliary electrodes, respectively. The ferrocenium/ferrocene couple was used as an internal standard. The reference electrode was Ag/Ag+ (0.1 M of AgNO3 in CH2Cl2). The CV measurements were carried out in anhydrous and nitrogen-saturated CH2Cl2 solution with 0.1 mol of n-tetrabutylammonium perchlorate (TBAP) and 1.0 mmol of analyte at a scan rate of 0.1 V s−1. UV-vis absorption spectra were recorded with a Perkin-Elmer Lambda 45 UV/vis spectrophotometer. Photoluminescence spectra at both 298 K and 77 K were recorded on an Edinburgh Analytical instrument FLS980. The lifetimes at different temperatures were determined on an Edinburgh analytical instrument FLS980 with a picosecond laser diode. The PL quantum yields were defined as the number of photons emitted per photon absorbed by the systems and measured on Edinburgh analytical instrument FLS920 equipped with an integrating sphere. The film samples in photophysical studies were prepared through spin-coating of a mixture of Cu(I) complexes (10 wt%) and PMMA (90 wt%) at 1000 rpm.Synthesis
[Cu(phpzpy)(POP)]BF4 (1). 1H NMR (400 MHz, CDCl3): δ 8.09 (d, 1H), 7.92 (m, 2H), 7.8 (d, 1H), 7.48 (t, 1H), 7.33–7.27 (m, 7H), 7.24–7.07 (m, 12H), 6.98–6.86 (m, 9H), 6.79 (dd 4H), 6.76–6.62 (m, 2H). 31P NMR: −12.78 (s). Elemental analysis: calc for C50H39BCuF4N3OP2: C, 65.99; H, 4.3; N, 4.62. Found: C, 65.44; H, 4.28; N, 4.49%.
[Cu(2-mphpzpy) (POP)]BF4 (2). 1H NMR (400 MHz, CDCl3): δ 8.19 (d, 1H), 7.96 (t, 1H), 7.83 (d, 1H), 7.68 (d, 1H), 7.52 (m, 2H), 7.39–7.26 (m, 8H), 7.20–7.12 (m, 8H), 7.06 (d, 2H), 6.96–6.90 (m, 4H), 6.73 (m, 7H), 6.52–6.49 (m, 2H), 6.18 (d, 1H), 1.69 (s, 3H). 31P NMR: −13.61 (s). Elemental analysis: calc for C56H42BCuF4N4OP2: C, 66.22; H, 4.44; N, 4.54. Found: C, 66.47; H, 4.59; N, 4.46%.
[Cu(2-tfphpzpy) (POP)]BF4 (3). H NMR (400 MHz, CDCl3): δ 8.16 (d, 1H), 7.96 (t, 1H), 7.89 (d, 2H), 7.83 (d, 1H), 7.74 (d, 1H), 7.4–7.31 (m, 8H), 7.26–7.12 (m, 9H), 7.02–6.91 (m, 5H), 6.83–6.75 (m, 7H), 6.58–6.51 (m, 2H), 6.18 (d, 1H). 31P NMR: −13.22 (s). Elemental analysis: calc for C56H42BCuF4N4OP2: C, 62.6; H, 3.91; N, 4.29. Found: C, 62.36; H, 4.28; N, 4.41%.
[Cu(4-tfphpzpy) (POP)]BF4 (4). H NMR (400 MHz, CDCl3): δ 8.21 (d, 1H), 8.10 (d, 1H), 7.99 (d, 1H), 7.94 (t, 1H), 7.45 (m, 4H), 7.39 (d, 1H), 7.36–7.30 (m, 5H), 7.26 (d, 1H), 7.20 (t, 4H), 7.10 (t, 4H), 7.05 (t, 1H), 7.00 (t, 4H), 6.91–6.83 (m, 8H), 6.75–6.71 (m, 2H). 31P NMR: −12.45 (s). Elemental analysis: calc for C56H42BCuF4N4OP2: C, 62.6; H, 3.91; N, 4.29. Found: C, 62.3; H, 3.97; N, 4.17%.
X-ray crystallographic analysis
Diffraction data of complexes 1 and 3 were collected on a SuperNova, Dual, Cu at zero, Atlas diffractometer equipped with graphite-monochromated Cu Kα radiation (λ = 1.54184 Å). Diffraction data of complexes 2 and 4 were collected on a Rigaku Mercury diffractometer equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Structures were solved with direct methods and refined with full-matrix least-squares methods with SHELXL-97 program package. Hydrogen atoms were added in idealized positions. All nonhydrogen atoms were refined anisotropically. Details of crystal and structure refinement were listed in Table S2.† Selected bond length and bond angles were listed in Table S3.†Device fabrication and characterization
The hole-blocking material bis[2-(di(phenyl)phosphino)-phenyl]ether oxide (DPEPO) was prepared according to literature, and purified through sublimation after recrystallization.62 Poly(3,4-ethylene dioxythiophene):poly(styrene sulfonic acid) (PEDOT:PSS) was purchased from Heraeus and filtered with 0.22 m filter before use. Other materials used in the device fabrication were purchased and used without further purification. A 40 nm thick PEDOT:PSS film was first spin-coated (at 3000 rpm) on a pre-cleaned ITO-glass substrate and then dried at 140 °C for 20 min. The emitting layer was then overlaid with spin-coating (at 1500 rpm) of CH2Cl2 solution with host and dopant (1 mg of 2 and 9 mg of PYD2 dissolved in 1.8 mL CH2Cl2). The film was then dried under vacuum for 1 h at room temperature. Subsequently, 50 nm of DPEPO, 0.8 nm of LiF and 100 nm of Al were deposited under pressure less than 4 × 10−4 Pa. The electroluminescence (EL) spectra were recorded on a HORIBA Jobin-Yvon FluoroMax-4 spectrometer. Current density–voltage–brightness (I–V–B) curves of these devices were recorded on a Keithley 2400/2000 source meter and a calibrated silicon photodiode at room temperature in air.Acknowledgements
This work was supported by the 973 key program of the Chinese Ministry of Science and Technology (MOST) (2012CB821705), the Chinese Academy of Sciences (KJCX2-YW-319, KJCX2-EW-H01), the National Natural Science Foundation of China (21373221, 21221001, 91122027, 51172232) and the Natural Science Foundation of Fujian Province (2006L2005).Notes and references
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS PubMed.
- B. Minaev, G. Baryshnikov and H. Agren, Phys. Chem. Chem. Phys., 2014, 16, 1719–1758 RSC.
- S. Schmidbauer, A. Hohenleutner and B. Konig, Adv. Mater., 2013, 25, 2114–2129 CrossRef CAS PubMed.
- B. Mi, H. Wang, Z. Gao, X. Wang, R. Chen and W. Huang, Progr. Chem., 2011, 23, 136–152 CrossRef CAS PubMed.
- D. G. Cuttell, S.-M. Kuang, P. E. Fanwick, D. R. McMillin and R. A. Walton, J. Am. Chem. Soc., 2002, 124, 6–7 CrossRef CAS PubMed.
- S. H. Chang, C. F. Chang, J. L. Liao, Y. Chi, D. Y. Zhou, L. S. Liao, T. Y. Jiang, T. P. Chou, E. Y. Li, G. H. Lee, T. Y. Kuo and P. T. Chou, Inorg. Chem., 2013, 52, 5867–5875 CrossRef CAS PubMed.
- B. S. Du, J. L. Liao, M. H. Huang, C. H. Lin, H. W. Lin, Y. Chi, H. A. Pan, G. L. Fan, K. T. Wong, G. H. Lee and P. T. Chou, Adv. Funct. Mater., 2012, 22, 3491–3499 CrossRef CAS PubMed.
- C. H. Lin, C. W. Hsu, J. L. Liao, Y. M. Cheng, Y. Chi, T. Y. Lin, M. W. Chung, P. T. Chou, G. H. Lee, C. H. Chang, C. Y. Shih and C. L. Ho, J. Mater. Chem., 2012, 22, 10684–10694 RSC.
- G.-G. Shan, H.-B. Li, D.-X. Zhu, Z.-M. Su and Y. Liao, J. Mater. Chem., 2012, 22, 12736–12744 RSC.
- C. L. Ho, H. Li and W. Y. Wong, J. Organomet. Chem., 2014, 751, 261–285 CrossRef CAS PubMed.
- T. Giridhar, C. Saravanan, W. Cho, Y. G. Park, J. Y. Lee and S. H. Jin, Chem. Commun., 2014, 50, 4000–4002 RSC.
- G. P. Tan, S. M. Chen, N. Sun, Y. H. Li, D. Fortin, W. Y. Wong, H. S. Kwok, D. G. Ma, H. B. Wu, L. X. Wang and P. D. Harvey, J. Mater. Chem. C, 2013, 1, 808–821 RSC.
- Q. L. Xu, C. C. Wang, T. Y. Li, M. Y. Teng, S. Zhang, Y. M. Jing, X. Yang, W. N. Li, C. Lin, Y. X. Zheng, J. L. Zuo and X. Z. You, Inorg. Chem., 2013, 52, 4916–4925 CrossRef CAS PubMed.
- E. Turner, N. Bakken and J. Li, Inorg. Chem., 2013, 52, 7344–7351 CrossRef CAS PubMed.
- K. Li, G. Cheng, C. S. Ma, X. G. Guan, W. M. Kwok, Y. Chen, W. Lu and C. M. Che, Chem. Sci., 2013, 4, 2630–2644 RSC.
- M. P. Gullo, J. B. Seneclauze, B. Ventura, A. Barbieri and R. Ziessel, Dalton Trans., 2013, 42, 16818–16828 RSC.
- H. Yersin, R. Czerwieniec and A. Hupfer, Organic Photonics V, 2012, 8435, 843508 Search PubMed.
- B. P. Rand, H. Yersin, R. Czerwieniec, A. Hupfer, C. Adachi and V. van Elsbergen, spie, 2012, 8435, 843508 CrossRef PubMed.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS PubMed.
- Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706–14709 CrossRef CAS PubMed.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
- H. Nakanotani, K. Masui, J. Nishide, T. Shibata and C. Adachi, Sci. Rep., 2013, 3(2127), 1–5 Search PubMed.
- M. Osawa, I. Kawata, R. Ishii, S. Igawa, M. Hashimoto and M. Hoshino, J. Mater. Chem. C, 2013, 1, 4375–4383 RSC.
- M. J. Leitl, F. R. Kuchle, H. A. Mayer, L. Wesemann and H. Yersin, J. Phys. Chem. A, 2013, 117, 11823–11836 CrossRef CAS PubMed.
- R. Czerwieniec, K. Kowalski and H. Yersin, Dalton Trans., 2013, 42, 9826–9830 RSC.
- M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson and H. Yersin, J. Am. Chem. Soc., 2014, 136, 16032–16038 CrossRef CAS PubMed.
- R. Czerwieniec, J. Yu and H. Yersin, Inorg. Chem., 2011, 50, 8293–8301 CrossRef CAS PubMed.
- L. Yang, J.-K. Feng, A.-M. Ren, M. Zhang, Y.-G. Ma and X.-D. Liu, Eur. J. Inorg. Chem., 2005, 1867–1879 CrossRef CAS PubMed.
- V. Kalsani, M. Schmittel, A. Listorti, G. Accorsi and N. Armaroli, Inorg. Chem., 2006, 45, 2061–2067 CrossRef CAS PubMed.
- M. Iwamura, S. Takeuchi and T. Tahara, J. Am. Chem. Soc., 2007, 129, 5248–5256 CrossRef CAS PubMed.
- T. J. Penfold, S. Karlsson, G. Capano, F. A. Lima, J. Rittmann, M. Reinhard, M. H. Rittmann-Frank, O. Braem, E. Baranoff, R. Abela, I. Tavernelli, U. Rothlisberger, C. J. Milne and M. Chergui, J. Phys. Chem. A, 2013, 117, 4591–4601 CrossRef CAS PubMed.
- N. Armaroli, G. Accorsi, F. Cardinali and A. Listorti, Top. Curr. Chem., 2007, 280, 69–115 CrossRef CAS.
- T. McCormick, W. L. Jia and S. Wang, Inorg. Chem., 2006, 45, 147–155 CrossRef CAS PubMed.
- S.-M. Kuang, D. R. McMillin, P. E. Fanwick and R. A. Walton, Inorg. Chem., 2002, 41, 3313–3322 CrossRef CAS PubMed.
- L. Zhang, B. Li and Z. Su, Langmuir, 2009, 25, 2068–2074 CrossRef CAS PubMed.
- D. Volz, M. Nieger, J. Friedrichs, T. Baumann and S. Brase, Langmuir, 2013, 29, 3034–3044 CrossRef CAS PubMed.
- X.-L. Chen, R. Yu, Q.-K. Zhang, L.-J. Zhou, X.-Y. Wu, Q. Zhang and C.-Z. Lu, Chem. Mater., 2013, 25, 3910–3920 CrossRef CAS.
- X.-L. Chen, C.-S. Lin, X.-Y. Wu, R. Yu, T. Teng, Q.-K. Zhang, Q. Zhang, W.-B. Yang and C.-Z. Lu, J. Mater. Chem. C, 2014, 3, 1187–1195 RSC.
- J. C. Deaton, S. C. Switalski, D. Y. Kondakov, R. H. Young, T. D. Pawlik, D. J. Giesen, S. B. Harkins, A. J. Miller, S. F. Mickenberg and J. C. Peters, J. Am. Chem. Soc., 2010, 132, 9499–9508 CrossRef CAS PubMed.
- M. Hashimoto, S. Igawa, M. Yashima, I. Kawata, M. Hoshino and M. Osawa, J. Am. Chem. Soc., 2011, 133, 10348–10351 CrossRef CAS PubMed.
- Z. Liu, M. F. Qayyum, C. Wu, M. T. Whited, P. I. Djurovich, K. O. Hodgson, B. Hedman, E. I. Solomon and M. E. Thompson, J. Am. Chem. Soc., 2011, 133, 3700–3703 CrossRef CAS PubMed.
- J. H. Min, Q. S. Zhang, W. Sun, Y. X. Cheng and L. X. Wang, Dalton Trans., 2011, 40, 686–693 RSC.
- S. Igawa, M. Hashimoto, I. Kawata, M. Yashima, M. Hoshino and M. Osawa, J. Mater. Chem. C, 2013, 1, 542–551 RSC.
- L. Bergmann, J. Friedrichs, M. Mydlak, T. Baumann, M. Nieger and S. Brase, Chem. Commun., 2013, 49, 6501–6503 RSC.
- K. J. Lotito and J. C. Peters, Chem. Commun., 2010, 46, 3690–3692 RSC.
- V. A. Krylova, P. I. Djurovich, M. T. Whited and M. E. Thompson, Chem. Commun., 2010, 46, 6696–6698 RSC.
- V. A. Krylova, P. I. Djurovich, B. L. Conley, R. Haiges, M. T. Whited, T. J. Williams and M. E. Thompson, Chem. Commun., 2014, 50, 7176–7179 RSC.
- D. M. Zink, M. Bachle, T. Baumann, M. Nieger, M. Kuhn, C. Wang, W. Klopper, U. Monkowius, T. Hofbeck, H. Yersin and S. Brase, Inorg. Chem., 2013, 52, 2292–2305 CrossRef CAS PubMed.
- D. M. Zink, T. Baumann, J. Friedrichs, M. Nieger and S. Brase, Inorg. Chem., 2013, 52, 13509–13520 CrossRef CAS PubMed.
- M. Osawa, M. Hoshino, M. Hashimoto, I. Kawata, S. Igawa and M. Yashima, Dalton Trans., 2015 10.1039/c4dt02853h.
- Z. W. Liu, J. Qiu, F. Wei, J. Q. Wang, X. C. Liu, M. G. Helander, S. Rodney, Z. B. Wang, Z. Q. Bian, Z. H. Lu, M. E. Thompson and C. H. Huang, Chem. Mater., 2014, 26, 2368–2373 CrossRef CAS.
- D. Volz, A. F. Hirschbiel, D. M. Zink, J. Friedrichs, M. Nieger, T. Baumann, S. Bräse and C. Barner-Kowollik, J. Mater. Chem. C, 2014, 2, 1457–1462 RSC.
- L. W. Qisheng Zhang, Adv. Mater., 2004, 16, 432–436 CrossRef PubMed.
- Q. Zhang, J. Ding, Y. Cheng, L. Wang, Z. Xie, X. Jing and F. Wang, Adv. Funct. Mater., 2007, 17, 2983–2990 CrossRef CAS PubMed.
- W. L. Jia, T. McCormick, Y. Tao, J. P. Lu and S. Wang, Inorg. Chem., 2005, 44, 5706–5712 CrossRef CAS PubMed.
- Q. S. Zhang, T. Komino, S. P. Huang, S. Matsunami, K. Goushi and C. Adachi, Adv. Funct. Mater., 2012, 22, 2327–2336 CrossRef CAS PubMed.
- J. L. Chen, X. F. Cao, J. Y. Wang, L. H. He, Z. Y. Liu, H. R. Wen and Z. N. Chen, Inorg. Chem., 2013, 52, 9727–9740 CrossRef CAS PubMed.
- A. Wada, Q. S. Zhang, T. Yasuda, I. Takasu, S. Enomoto and C. Adachi, Chem. Commun., 2012, 48, 5340–5342 RSC.
- C. W. Hsu, C. C. Lin, M. W. Chung, Y. Chi, G. H. Lee, P. T. Chou, C. H. Chang and P. Y. Chen, J. Am. Chem. Soc., 2011, 133, 12085–12099 CrossRef CAS PubMed.
- G. J. Kubas, Inorg. Synth., 1979, XIX, 90–92 CrossRef PubMed.
- Z. R. Reeves, K. L. V. Mann, J. C. Jeffery, J. A. McCleverty, M. D. Ward, F. Barigelletti and N. Armaroli, J. Chem. Soc., Dalton Trans., 1999, 349–356 RSC.
- H. Xu, L. H. Wang, X. H. Zhu, K. Yin, G. Y. Zhong, X. Y. Hou and W. Huang, J. Phys. Chem. B, 2006, 110, 3023–3029 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary computational and experimental data. CCDC 1041001–1041004. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra04591f |
| This journal is © The Royal Society of Chemistry 2015 |