Cancer cell extinction through a magnetic fluid hyperthermia treatment produced by superparamagnetic Co–Zn ferrite nanoparticles†
Raghvendra A. Boharaa,
Nanasaheb D. Thoratab,
Akhilesh K. Chaurasiab and
Shivaji H. Pawar*a
aCentre for Interdisciplinary Research, D.Y. Patil University, Kolhapur-416006, India. E-mail: raghvendrabohara@gmail.com; shpawar1946@gmail.com; Fax: +91-0231-2601595; Tel: +91-0231-260122
bSamsung Biomedical Research Institute, Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon 440-746, South Korea
First published on 6th May 2015
Abstract
Cobalt zinc ferrite (CZF) magnetic nanoparticles (MNPs) were synthesized by modifying a thermal decomposition method in the presence of triethylene glycol (TEG). Initially structural, morphological, and magnetic characterizations were carried out in order to confirm their size, polydispersity, colloidal stability, and magnetic property. Fourier transform infrared spectroscopy (FTIR) confirmed the presence of triethylene glycol (TEG) on the surface of CZF MNPs. The CZF MNPs has revealed a superparamagnetic nature with high saturation magnetization, good colloidal stability, high specific absorption rate (SAR), excellent biocompatibility, and a monodispersed nature. All these properties are crucial, for their use as a nanomedicine in magnetic fluid hyperthermia (MFH) treatment; which is considered to be one of the most promising cancer therapies. The prepared CZF MNPs are found to be biocompatible with MCF7 (human breast cancer) and L929 (mouse fibroblast) cell lines, when tested by MTT and SRB assays. Cell particle interaction was examined in depth, by using multiple staining techniques coupled with confocal microscopy. Finally, an in vitro hyperthermia experiment was carried out on MCF7 cells, resulting in the extinction of MCF7 cells by up to 80% within 60 min. The nature of the cell extinction was found and lastly reactive oxygen species (ROS) production was assessed, where ROS is the responsible factor for apoptosis. This research demonstrates that, prepared CZF MNPs can be used as a potential candidate for effective MFH treatment for cancer cell extinction.
1 Introduction
Recent developments in nanotechnology have provided a new set of systems, methodologies and materials for biomedical research and applications. The advantage of nanomaterials is that, they provide a large surface area, offering a better interaction with different biological entities.1,2 Among the different nanomaterials, magnetic nanoparticles (MNPs), owing to their superparamagnetic behaviour, are promising candidates for biomedical applications, such as a contrast agent in magnetic resonance imaging (MRI), drug delivery, detection of pathogens, enzyme immobilization, and magnetic fluid hyperthermia (MFH).3,4 Among these, MFH treatment by using MNPs has emerged to be one of the most promising cancer modalities, that can be used either alone or in combination with chemotherapy and radiotherapy. In MFH treatment with MNPs, heat is applied to the specific region of the body or tissue via absorption of radio/micro frequency when exposed to an alternating current (AC) magnetic field.5 For effective clinical applications of MNPs via MFH, they should possess a large specific absorption rate (SAR), biocompatibility, and should form a stable suspension in physiological media like water and phosphate buffer (PBS). The SAR of MNPs strongly depends on magnetic moment, anisotropic energy, density, particle size and size distribution.6 Among various MNPs, ferrites (Fe3O4) have been mostly preferred for MFH treatment, because of their biocompatibility, ease of synthesis and their corresponding development as a contrast agent in MRI. However, the Curie temperature (Tc) of Fe3O4 is 823 K (550 °C), which is much superior to hyperthermia temperature. When such nanoparticles of Fe3O4 are subjected to an AC magnetic field for MFH, because of high Tc they can attain a temperature of 100–300 °C depending on the frequency (f), magnetic field (H) and duration (t). This may be harmful to normal tissues and cells and may cause serious consequences.7 To overcome this drawback of ferrites, spinel ferrites can be considered for MFH. Among various spinel ferrites, zinc substituted cobalt ferrites appear to be a potential candidate for hyperthermia, since it has desired Tc which is 415 K. In addition to this, one can tune its magnetic properties like magnetization, coercive force to the values, ensuring a reasonable heating efficiency and the self-controlled heating mechanism.8 Many methods have been developed for synthesis of mixed ferrites such as co-precipitation, ceramic technique, and low temperature combustion.9–12 In our recent publication, we have synthesized amine functionalized Co0.5Zn0.5Fe2O4 MNPs by high thermal decomposition method.13 Following this, here we have synthesized cobalt zinc ferrite (Co0.5Zn0.5Fe2O4) (CZF) by high thermal decomposition method with slight modification. Typically high thermal decomposition method includes decomposition of metal precursor in high boiling temperature solvents, in the presence of stabilizing agents. However, obtained MNPs are highly organic soluble, which are difficult to suspend in water. Therefore, further surface modification is necessary to optimize their water suspension stability. It has been seen that various polymers are used to convert hydrophobicity to hydrophilicity, but obtained nanoparticles from these surface modification processes cannot be used for long term application as there might be a risk of dissociation of layers, which restricts their use in most of biomedical applications.14,15 Therefore, in the present experiment, we have used triethylene glycol (TEG) during synthesis of CZF MNPs, which are hydrophilic in nature. This method is developed in such way that the prepared MNPs do not require any further process to modify the surface. This study reports a reproducible technique for the preparation of a TEG mediated synthesis of superparamagnetic CZF MNPs which are highly water dispersible. Surface modification was done in one-step, thus avoiding frequent exposure to multistep surface modification methods that may cause decrease in magnetic property of the material. The prepared CZF MNPs were characterized for required physical, morphological and biocompatible nature. Cell particle interactions were found out in depth, by multiple staining techniques, on confocal laser scanning microscope (CLSM). The hyperthermia effect of the CZF MNPs under the oscillating magnetic field was studied. After that, the prepared MNPs were used for in vitro hyperthermia applications on MCF7 cell line.2 Experimental
2.1 Materials
FeCl3, CoCl2, and ZnCl2 and Na acetate were purchased from Molychem India. Triethylene glycol (TEG) was purchased from Thomas Baker. All chemicals used here were of analytical grade and used without further purification.2.2 Synthesis of TEG stabilized CZF MNPs
The water dispersible CZF MNPs were prepared by modified high thermal decomposition method.13 To the mixture of CoCl2 (1.5 mmol), ZnCl2 (1.5 mmol) and FeCl3 (3.00 mmol) dissolved in 40 mL TEG, sodium acetate was added and heated to 110 °C under vigorous magnetic stirring. After heating for 1 h, the reaction mixture was refluxed at 210 °C for 2 h and further 2 h more at 295 °C during which fine black color colloidal particles appeared. The reaction mixture was allowed to cool down to room temperature. The particles were washed several times with mixture of ethanol and ethyl acetate, separated magnetically, and dried in hot air oven at 60 °C for 2 h.2.3 Characterizations
t = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
| H = 1.257ni/L in Oe | (2) |
2.3.2.1 Cell culture. The comparative in vitro cytotoxicity study of CZF MNPs was done on MCF7 and L929 cell lines. These cells were grown in DMEM (Dulbecco's Modified Eagle Medium) supplemented with 10% v/v fetal bovine serum, kanamycin (0.1 mg mL−1), penicillin-G (100 U mL−1), and sodium bicarbonate (1.5 mg mL−1) at 37 °C in a 5% CO2 atmosphere.
2.3.2.2 MTT assay. MCF7 and L929 cells were incubated at a concentration of 2 × 105 cells per mL, in their respective mediums for 24 h in a 96-well microtitre plates. After 24 h, the old media was replaced by fresh media and CZF MNPs with different concentration (0.5, 1.0, 1.5, and 2.0 mg mL−1 of culture media). The plates containing cells with variable amounts of CZF MNPs were incubated at 37 °C in a 5% CO2 atmosphere for 24 and 48 h, and were subjected to MTT assay. The detailed procedure has been mentioned in our earlier publication.16 The relative cell viability (%) were compared with control cells without CZF MNPs and calculated by the equation.
| [Absorbance] tested/[absorbance] control × 100 |
2.3.2.3 SRB assay. MCF7 cells were incubated at a concentration of 2 × 105 cells per mL for 24 h in a 96-well micro titter plate. After 24 h, the old media was replaced by fresh media and CZF MNPs with different concentration (0.5, 1.0, 1.5, and 2.0 mg mL−1 of culture media). The plates containing cells with variable amounts of CZF MNPs were incubated at 37 °C in a 5% CO2 atmosphere for 24 and 48 h, and were subjected to SRB assay with a procedure which has been mentioned in our earlier publications.13
2.3.2.4 Confocal microscopy study. MCF7 and L929 cells at a concentration of 2 × 105 cells per mL were grown separately in DMEM, and were transferred to the slide Petri dishes containing 2 mL of DMEM. The cells were kept in slide Petri dishes for 24 h for growth. The cell culture media were changed to new DMEM media, and different concentrations of 0, 0.2, 0.4, 0.6, 0.8 and 1 mg mL−1 of CZF MNPs were added in each type of cell line. Both MCF7 and L929 cells, with and without nanoparticles were incubated in a CO2 incubator for 24 h. The media were removed and, washed 3 times with PBS (pH 7.4). After washing, the cells were stained with 4,6-diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC), and propidium iodide (PI) (1 μg mL−1) for 5 min. Then the stained cells were washed with PBS. Finally, cells were overlaid with 400 μL PBS and directly observed (without cell fixation) under a confocal microscope (at 40× magnification Zeiss LSM 510 Meta). Blue, green and red fluorescent cells were observed respectively, by excitation and emission, DAPI (λ excitation 358 nm, λ emission 461 nm), FITC (λ excitation 495 nm, λ emission 519 nm), and PI (λ excitation 535 nm, λ emission 617 nm), and detected with a band-pass filter with the final images generated by superimposing the blue, green and red image as described earlier.16
3 Results and discussion
3.1 Structural analysis
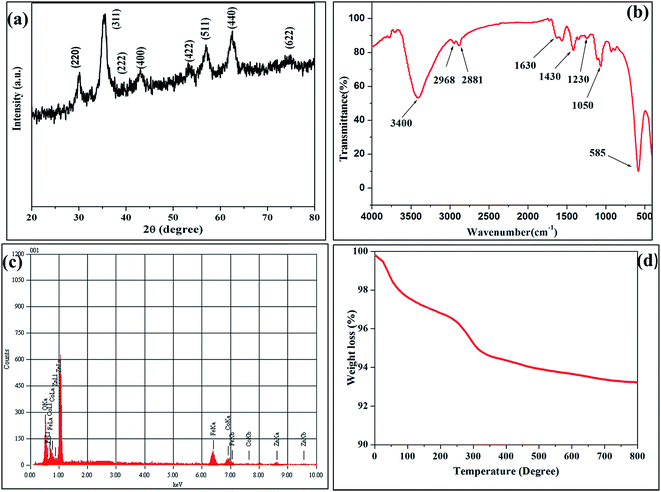
The room temperature X-ray diffraction pattern of the CZF samples is shown in Fig. 1(a). All XRD peaks were matched with JCPDS Card no. 22-1086 of the (CoFe2O4) and JCPDS Card no. 22-1012 of the (ZnFe2O4). The lattice parameters were calculated from the reflection of (311) plane using standard formula and found to be 0.842 nm. The lattice parameter of CZF MNPs was higher than lattice parameter of cobalt ferrite (CoFe2O4). The reason behind this is the diffusion of zinc ions into tetrahedral sites, which leads to increase in lattice parameter of CZF MNPs.17 The (311) peak was chosen for calculating the average particle size of the sample using the Scherrer formulae. The result shows that the crystallite size was ∼12 nm, also broadening of the peaks suggest nanocrystalline nature of the sample. Fig. 1(b) shows FTIR spectra of CZF MNPs which clearly shows the presence of the TEG molecule present on the surface. The presence of a strong absorption band at about 585 cm−1 corresponds to metal–oxygen stretching vibration. The peaks at about 2968–2881, 1630, 1430, 1230 and 1050 cm−1 are due to C–H stretching, O–H stretching, C–H bending, C–O bending and O–H bending vibrations respectively, thus confirming the presence of TEG molecule on the particle surface.18 The broad band between 3400 cm−1 corresponds to O–H stretching vibrations ascribed for water and TEG molecule presence on the surface. The EDS spectra were used as a quantitative elemental analysis of CZF MNPs, shown in Fig. 1(c). The corresponding peaks in spectrum were due to Co, Fe, Zn and O only and do not show any additional impurity peak implying purity of the samples. The presence of TEG on the surface of the MNPs is also supported by TGA analysis which is shown in Fig. 1(d). As TGA is performed under flowing N2, the oxidation of MNPs surface was greatly reduced. The graph shows two-stage decomposition profile, where the initial weight loss correspondence to removal of surface adsorbed moisture. The later weight loss which is most significant one, ascribed to the removal of TEG molecules present on the surface. The presences of these TEG molecules on the surface are accounted for good water dispersibility which is most requisite for biomedical applications.3.2 Morphological characterization
The size and shapes of the MNPs were observed by using TEM micrographs. Fig. 2(a) and (b) shows the TEM micrograph of synthesized CZF MNPs. From micrographs, it can be seen that particles are uniform in size and spherical in shape. The average particle size is within the range ∼15 nm and matches with the crystallite size calculated from the XRD pattern. Fig. 2(c) shows HR-TEM micrographs of the sample. The lattice fringe spacing of the sample was measured to be 0.27 nm and which corresponds (311) plane of CZF MNPs.3.3 Colloidal stability and magnetic study of CZF MNPs
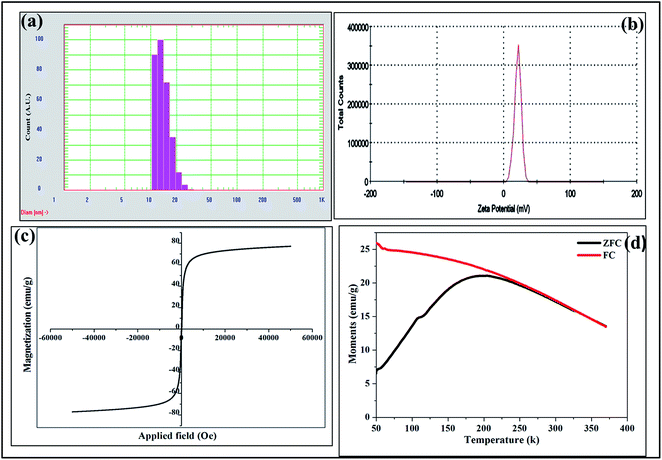
It is always important to study the colloidal stability of MNPs as this property has vital role to play when these particles are to be used as nanomedicine.19 DLS and zeta potential techniques were used to identify the colloidal stability of CZF MNPs in aqueous medium. The DLS measurement of CZF MNPs is shown in Fig. 3(a). The average maximum of DLS size for CZF MNPs gives the average hydrodynamic diameter (HDD). It has been observed that the HDD shows a narrow size distribution between 13 and 30 nm with a poly dispersity of 0.2, which suggests the particles are monodispersed in nature. Fig. 3(b) shows the zeta potential distribution plot and the maximum of the plot is at about +21 mV, which indicates that the surface of nanoparticles is positively charged. The TEG molecules present on the surface may polarizes into R–O− and H+ ions at high temperature, and this H+ is accountable for positive surface and enhanced water dispersibility. The particle remains stable in colloidal solution due to electrostatic and steric interaction between the MNPs and can be the reason for water stability and which is most wanted in biomedical applications. The magnetic properties of CZF MNPs are shown in Fig. 3(c), which is measured by magnetization as a function of field and temperature and obtained results are discussed below. From the figure, it can be seen that the Ms value of the CZF MNPs is 76.53 emu g−1 at an applied of ±40 kOe. Further magnetization measurements showed no coercivity and no remanence in the absence of the external field which indicates that the particles are superparamagnetic at room temperature (300 K).20 The temperature dependent magnetization (FC–ZFC) of the CZF MNPs is measured in the applied magnetic field of 100 Oe and recorded in zero-filed-cooling (ZFC) and filed cooling (FC) and obtained results are shown in Fig. 3(d). The ZFC curve reached maximum at 200 K, which corresponds to the blocking temperature of the sample (TB). Above this, sample shows superparamagnetic nature. However, the superimposition of FC–ZFC takes place at 278 K. The superimposition of FC–ZFC curves is the specific feature for superparamagnetic system and which can be considered as valuable property for hyperthermia applications.21 Therefore, the current synthesis technique enables us to synthesize superparamagnetic nanoparticles with high magnetic saturation values.3.4 Biocompatibility and cell particle interaction study of CZF MNPs
3.5 Induction heating study of CZF MNPs
For effective in vivo administration, it is necessary that the nanoparticles should produce a maximum temperature rise at the lowest concentration. It has been seen that for superparamagnetic nanoparticles the greatest relaxation losses are due to Brownian modes (heat due to friction arising from total particle oscillations) and Neel modes (heat due to rotation of the magnetic moment with each field oscillation). The calculations for heat dissipation by superparamagnetic nanoparticles can be found under ESI.† The induction heating ability of MNPS was tested at different currents (200–400 A i.e. 167–335 Oe) for 10 min, with the variation of particle concentration of 1, 2, and 5 mg mL−1 in water. Fig. 6(a) and (b) represent the temperature kinetic curves for 2 and 5 mg mL−1 concentration of CZF MNPs dispersed in water respectively, obtained after application of an alternating magnetic field. It is observed from induction heating experiment that, hyperthermia temperature (42–43 °C) was achieved by 1 and 2 mg mL−1 sample concentration at magnetic fields of 335 Oe and at a frequency of 267 kHz. However, in the case of 5 mg mL−1 the hyperthermia temperature was achieved at all filed with the same frequency. The more distinct picture of hyperthermia measurements got clear after the detailed study of SAR. The heating capacity of a magnetic material is quantified through the SAR, defined as the amount heat generated per unit gram of magnetic material per unit time. SAR values were calculated by using the relation (7) (see in ESI†). Regarding, 1 mg mL−1 concentration, SAR values are motivating and increased from 38 to 132 W g−1 when AC magnetic field increases from 167.6 Oe to 335.2 Oe. It is fascinating to note that, though there is a higher temperature rise in the case of 5 mg mL−1 as compared to 1 and 2 mg mL−1 sample concentration, the value of SAR is almost equal in the case of 1 mg mL−1 and higher in case of 2 mg mL−1 [Fig. 6(c)]. This perhaps might occur due to high particle concentration resulting into particle agglomeration, leading into the intense rise in the interparticles dipolar interaction and less power dissipation to the medium. The energy dissipation mechanism strongly depends on the dipole–dipole interaction.27,283.6 In vitro magnetic fluid hyperthermia study of CZF MNPs
The efficacy of CZF MNPs in the killing of MCF7 (human breast cancer) cell line by in vitro hyperthermia was determined by a MTT assay and the obtained results are shown in Fig. 6(d). During the experiment, the temperature of the CZF MNPs was maintained at 42–43 °C. The cells with CZF MNPs were heated for different time intervals of 10, 20, 30, 45, and 60 min and results were measured after 1 h and 24 h for proper understanding. The exposure of magnetic field to MCF7 cells in the absence of CZF MNPs had not shown any effect on cell viability, suggesting that the ac magnetic field used in the study is safe. It has been seen that, 10 min exposure leads the killing efficiency to 48.89% and 49.02%, when measured after 1 h and 24 h respectively. As exposure time was increased, it has seen that there is increase in killing efficiency. In addition, it is also found that, in the case of 60 min exposure, the % killing for 1 h was 66.81% and for 24 h it was 82.51%. To validate the observed results and to find out the nature of the cell death, the cells were examined by staining with DAPI, FITC, and PI dyes, by confocal microscopy. Fig. 7(a) shows confocal images of CZF MNPs treated and untreated MCF7 cells, after MFH for 10 and 60 min, which are stained with DAPI, FITC, and PI after 1 h incubation. Before application of MFH, the cells are uniformly stained with DAPI and FITC. But after exposure to MFH treatment, there is a time dependent increase in PI staining of cells, while FITC staining is disrupted. This allows the PI to enter inside the cells, staining the nucleus of them, which confirms the cell death due to apoptosis as per previous report.293.7 ROS generation during magnetic fluid hyperthermia
Reactive oxygen species (ROS) are broadly defined as oxygen containing reacting species, which are biologically important and perform various vital role like cell signalling.30 It has been seen that regulated levels of ROS play a vital role in cancer cell proliferation and any change in their level may lead to apoptosis and cell death. Therefore, upsetting ROS haemostasis can be as encouraging approach for killing cancer cells.31 It is reported that, MNPs can induce ROS.32,33 Here we have investigated that ROS generation might be a possible mechanism for cancer cell killing with MFH therapy. Fig. 7(b) represents ROS assay, where a time dependent increased levels of ROS is found, keeping CZF MNPs as a control. This is mainly attributed to MFH therapy, which induce heat mediated oxidative stress, bringing imbalance between destructive oxidants (ROS) and defensive antioxidants, and resulting into major cellular damage via induction of genotoxicity, inflammation and damage to critical biomolecules such as DNA, proteins, and lipids. All this triggers the apoptosis pathway and ultimately killing of cancer cells.34–36 Thus, our findings demonstrate that MFH treatment induces significant ROS production that is responsible for killing of cancer cells.4 Conclusion
CZF MNPs synthesized by facile one step method are found to be highly water dispersible, monodispersed and superparamagnetic in nature. The synthesis method is cost-effective, easy to scale up and reproducible. The TEM micrographs reveal spherical shape of particles, having diameter of ∼15 nm. The presence of TEG on surface of CZF MNPs was confirmed by FTIR and TGA analysis. A high value of SAR (165 W g−1) was observed which may be due to high colloidal stability of CZF MNPs in aqueous media, attributed to presence of the TEG on the particle surface. The particles show excellent biocompatibility with different types of cell lines. MCF7 cells were extincted up to 80% within 60 min, when subjected for in vitro hyperthermia treatment by CZF MNPs. Confocal images and ROS study of post hyperthermia treated cells affirm cell extinction by apoptosis. From all these observations, it is concluded that, CZF MNPs can be used as a potential candidate for effective MFH treatment for cancer cell extinction.Acknowledgements
Authors are very grateful to Prof. S. K. Dhar, TIFR, Mumbai for M–T measurement, SAIF-NEHU, Shillong for TEM facility. The authors also acknowledge SAIF Kochi for FTIR facilit.References
- K. E. Sapsford, W. R. Algar, L. Berti, K. B. Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904–2074 CrossRef CAS PubMed.
- M. P. Monopoli, C. Aberg, A. Salvati and K. A. Dawson, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- S.-N. Sun, C. Wei, Z.-Z. Zhu, Y.-L. Hou, S. S. Venkatraman and Z.-C. Xu, Chin. Phys. B, 2014, 23, 037503 CrossRef.
- K. Hola, Z. Markova, G. Zoppellaro, J. Tucek and R. Zboril, Biotechnol. Adv., 2015 DOI:10.1016/j.biotechadv.2015.02.003.
- A. Hervault and N. T. K. Thanh, Nanoscale, 2014, 6, 11553–11573 RSC.
- I. Sharifi, H. Shokrollahi and S. Amiri, J. Magn. Magn. Mater., 2012, 324, 903–915 CrossRef CAS.
- R. Epherre, E. Duguet, S. Mornet, E. Pollert, S. Louguet, S. Lecommandoux, C. Schatz and G. Goglio, J. Mater. Chem., 2011, 21, 4393 RSC.
- S. Amiri and H. Shokrollahi, Mater. Sci. Eng., C, 2013, 33, 1–8 CrossRef CAS PubMed.
- R. M. Patil, P. B. Shete, N. D. Thorat, S. V. Otari, K. C. Barick, A. Prasad, R. S. Ningthoujam, B. M. Tiwale and S. H. Pawar, J. Magn. Magn. Mater., 2014, 355, 22–30 CrossRef CAS.
- T. M. Meaz, S. M. Attia and A. M. A. El, J. Magn. Magn. Mater., 2003, 257, 296–305 Search PubMed.
- H. Y. He, J. Nanotechnol., 2011, 2011, 1–5 CrossRef.
- N. D. Thorat, K. P. Shinde, S. H. Pawar, K. C. Barick, C. A. Betty and R. S. Ningthoujam, Dalton Trans., 2012, 41, 3060–3071 RSC.
- R. A. Bohara, H. M. Yadav, N. D. Thorat, S. S. Mali, C. K. Hong, S. G. Nanaware and S. H. Pawar, J. Magn. Magn. Mater., 2015, 378, 397–401 CrossRef CAS.
- D. Maity, P. Chandrasekharan, C.-T. Yang, K.-H. Chuang, B. Shuter, J.-M. Xue, J. Ding and S.-S. Feng, Nanomedicine, 2010, 5, 1571–1584 CrossRef CAS PubMed.
- S. Mohapatra, S. R. Rout, S. Maiti, T. K. Maiti and A. B. Panda, J. Mater. Chem., 2011, 21, 9185 RSC.
- N. D. Thorat, S. V. Otari, R. M. Patil, R. A. Bohara, H. M. Yadav, V. B. Koli, A. K. Chaurasia and R. S. Ningthoujam, Dalton Trans., 2014, 43(46), 17343–17351 RSC.
- C. Hou, H. Yu, Q. Zhang, Y. Li and H. Wang, J. Alloys Compd., 2010, 491, 431–435 CrossRef CAS.
- D. Maity, S.-G. Choo, J. Yi, J. Ding and J. M. Xue, J. Magn. Magn. Mater., 2009, 321, 1256–1259 CrossRef CAS.
- N. D. Thorat, R. M. Patil, V. M. Khot, A. B. Salunkhe, A. I. Prasad, K. C. Barick, R. S. Ningthoujam and S. H. Pawar, New J. Chem., 2013, 37, 2733 RSC.
- N. D. Thorat, S. V. Otari, R. A. Bohara, H. M. Yadav, V. M. Khot, A. B. Salunkhe, M. R. Phadatare, A. I. Prasad, R. S. Ningthoujam and S. H. Pawar, Mater. Sci. Eng., C, 2014, 42, 637–646 CrossRef CAS PubMed.
- R. A. Bohara, N. D. Thorat, H. M. Yadav and S. H. Pawar, New J. Chem., 2014, 38, 2979 RSC.
- N. D. Thorat, S. V. Otari, R. M. Patil, V. M. Khot, A. I. Prasad, R. S. Ningthoujam and S. H. Pawar, Colloids Surf., B, 2013, 111, 264–269 CrossRef CAS PubMed.
- M. M. Song, W. J. Song, H. Bi, J. Wang, W. L. Wu, J. Sun and M. Yu, Biomaterials, 2010, 31, 1509–1517 CrossRef CAS PubMed.
- J. Blechinger, A. T. Bauer, A. A. Torrano, C. Gorzelanny, C. Bräuchle and S. W. Schneider, Small, 2013, 9, 3970–3980 CrossRef CAS PubMed.
- J. M. Clarke, M. R. Gillings, N. Altavilla and A. J. Beattie, J. Microbiol. Methods, 2001, 46, 261–267 CrossRef CAS PubMed.
- S.-J. Choi, J.-M. Oh and J.-H. Choy, J. Inorg. Biochem., 2009, 103, 463–471 CrossRef CAS PubMed.
- R. Hergt, S. Dutz and M. Zeisberger, Nanotechnology, 2010, 21, 015706 CrossRef PubMed.
- D. S. Nikam, S. V. Jadhav, V. M. Khot, M. R. Phadatare and S. H. Pawar, J. Magn. Magn. Mater., 2014, 349, 208–213 CrossRef CAS.
- D. S. Y. Qu, J. Li, J. Ren, J. Leng and C. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 16867–16879 Search PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- M. J. Hajipour, O. Akhavan and A. Meidanchi, RSC Adv., 2014, 4, 62557–62565 RSC.
- S. Alarifi, D. Ali, S. Alkahtani and M. S. Alhader, Biol. Trace Elem. Res., 2014, 159, 416–424 CrossRef CAS PubMed.
- T. Sadhukha, L. Niu, T. S. Wiedmann and J. Panyam, Mol. Pharm., 2013, 10, 1432–1441 CrossRef CAS PubMed.
- P. L. Apopa, Y. Qian, R. Shao, N. L. Guo, D. Schwegler-Berry, M. Pacurari, D. Porter, X. Shi, V. Vallyathan, V. Castranova and D. C. Flynn, Part. Fibre Toxicol., 2009, 6, 1 CrossRef PubMed.
- R. Franco, O. Schoneveld, A. G. Georgakilas and M. I. Panayiotidis, Cancer Lett., 2008, 266, 6–11 CrossRef CAS PubMed.
- T. Ozben, J. Pharm. Sci., 2007, 96, 2181–2196 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04553c |
| This journal is © The Royal Society of Chemistry 2015 |