Geopolymerization with bagasse bottom ash and china clay, effect of calcination temperature and silica to alumina ratio
Noor-ul-Amin*a,
Mahtab Faisalb,
Khan Muhammada and
Wajid Aminc
aDepartment of Chemistry, Abdul Wali Khan University, Mardan, Pakistan. E-mail: noorulamin_xyz@yahoo.com
bBuilding Materials Section, PCSIR Laboratories Complex, Peshawar, Pakistan
cDepartment of Physics, Kohat University of Science and Technology, Kohat, Pakistan
First published on 9th July 2015
Abstract
The object of this work to study geopolymerization using bagasse ash and china clay at the most appropriate calcination temperature and ratio of silica to alumina. Bagasse ash from the sugar industry and china clay from Shah Dheri Swat in Pakistan were used in this work. Both of the raw materials were characterized using XRF, FTIR and XRD. The clay was calcined between a temperature range of 600–1000 °C. The resulting amorphous material was reacted with bagasse ash and sodium silicate using different ratios of silica to alumina ranging from 2–4. The reaction was carried out in a strongly alkaline solution of sodium hydroxide to produce geopolymer cement whose compressive strength and setting time were studied. The geopolymer cement specimens were characterized using XRF, XRD and SEM. The highest compressive strength for the geopolymers of 16.64 MPa was obtained from the mix having a SiO2/Al2O3 ratio of 2.7 and clay calcined at a temperature of 900 °C.
1. Introduction
Presently Portland cement is the most commonly used of all hydraulic binders in the present era but its production is a highly energy intensive process, and additionally causes the release of high amounts of greenhouse gases into the environment.1,2 Portland cement production faces two basic problems, on one side, it needs 1450 °C in a cement kiln which is achieved by the burning of coal, gas or furnace oil, making the cost of production very high due to the high prices of fuel, while on the other side, huge amounts of greenhouse gases are released into the environment. The production of one ton of cement results in the release of approximately one ton of carbon dioxide, due both to the calcination of limestone and burning of fuel. Portland cement contains 60–65% CaO which is obtained by the calcination of CaCO3. In order to address these problems, researchers have been trying to design new binders alternative to existing cements, which would substitute Portland cement, and also be manufactured in such a way as to safeguard our environment. Compared with ordinary Portland cement, geopolymers are well-known binding materials because of their excellent characteristics, including their higher strength,3,4 low shrinkage,5 good acid resistance and fire resistance,3,5 comparatively low emission of greenhouse gases,3 high thermal resistivity,5 tremendous immobilization of heavy metals,6 high thermal stability,7 and low cost of production.8 Due to such properties, the potential applications of such materials in the construction industry include fire proofing,9 biomaterials10 and waste treatment.11Geopolymerization is a geo synthetic reaction of alumino-silicate minerals in a strongly alkaline medium at low temperature.12 Geopolymerization is a complex procedure which is not fully understood at the present time. A simple reaction mechanism involves the condensation polymerization of ortho-silicate ions which are considered to be hypothetical monomers. The mechanism proposed by Davidovits13 is as follows:
The equations above show the synthesis of a geopolymer having a silica to alumina ratio (Si/Al) = 1. This ratio may be different depending upon the compositions of the alumino-silicate material and alkaline activator. Usually, the geopolymerization process involves dissolution, transportation and polycondensation.14 It is anticipated that geopolymer gel may diffuse among the interstitial spaces of the particles. On the hardening of the gel, the separate alumino-silicate particles are consequently bound together, resulting in a matrix that possesses good mechanical properties, like compressive strength. In early research work, meta-kaolinite, (calcined kaolinite) was utilized as the main material in geopolymerization reactions.15,16
Geopolymerization is mainly divided into three steps, as reported by a number of researchers.17 In the first step, silica and alumina source materials are dissolved in alkaline solution, in the second step re-organization and dispersion of the dissolved ions takes place with the formation of small coagulated arrangements while the third step involves the poly-condensation of the soluble species and the formation of hydrated products.
Geopolymerization may be carried out through a sol–gel method, in which the most common raw material may be rich in alumina and silicate, like natural pozzolan, fly ash, blast furnace slag, and thermally activated kaolinite clays. On thermal activation the kaolinite is transformed into an anhydrous alumino-silicate which is the essential part of the geopolymer. On thermal activation the kaolinite is converted into a disordered structure, and possesses enormous reaction potential when treated in a strongly alkaline medium like sodium hydroxide18 or an aqueous solution of calcium hydroxide.10
Studying the literature on geopolymers, it becomes clear that a number of researchers have thermally treated kaolinite clays at different levels of temperature. Davidovits and Davidovits19 activated a kaolinite clay at temperatures of 500, 650, 700 and 750 °C and concluded that 750 °C was the optimum temperature for thermal activation. Palomo et al.20 reported that a 600–700 °C temperature range for clay calcination was optimum, while Cioffi et al.21 activated clay at 500, 550, 650 and 750 °C. Zhang and Sun-Wei22 used 700 °C, while Zibouche et al.23 converted kaolinite into meta-kaolinite clay at 800 °C. Chareerat et al.24 thermally activated a kaolinite clay at various temperatures between 400–800 °C at intervals of 100 °C for different durations of 2, 4 and 6 h and reported 600 °C as the best calcination temperature. The geopolymer was synthesized by employing a mixture of 20% meta-kaolinite and 80% ash. All these researchers synthesized geopolymers with different properties.
In the present work geopolymerization was performed using bagasse bottom ash, a waste of the sugar industry and china clay activated at different temperatures and different silica to alumina ratios, and the effects of these parameters on the properties of the geopolymer composites and mortar were studied. Moreover, the effect of alkali concentration on the geopolymer properties has also been reported in this paper. Geopolymer cement pastes were also characterized with XRD, FTIR and scanning electron microscopy.
2. Material and methods
2.1. Collection of raw materials
For the synthesis of the geopolymer, bagasse bottom ash, china clay, sodium silicate, sodium hydroxide and de-ionized water were used. Bagasse bottom ash was collected from the Premier Sugar mill, Mardan KPK, Pakistan. China clay was collected from Shah Dheri in the district of Swat, and sodium silicate was purchased from a local glass industry at the industrial estate Hayat Abad Peshawar. The chemical compositions of the bagasse ash and china clay are given in Table 1. Sodium hydroxide from the Merck Company was purchased in the local market. De-ionized water was obtained from the PCSIR Labs, Peshawar. A 3 M aqueous solution of sodium hydroxide and sodium silicate in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight was used as the basic solution. The sodium hydroxide solution was prepared by dissolving dried pellets of 99% pure sodium hydroxide in distilled water. An aqueous solution of sodium silicate was made with a composition of 28.7% SiO2, 8.9% Na2O and 62.4% H2O by weight.
1 by weight was used as the basic solution. The sodium hydroxide solution was prepared by dissolving dried pellets of 99% pure sodium hydroxide in distilled water. An aqueous solution of sodium silicate was made with a composition of 28.7% SiO2, 8.9% Na2O and 62.4% H2O by weight.
| Oxide | Weight (%) | |
|---|---|---|
| Bagasse bottom ash | China clay | |
| SiO2 | 78.046 | 46.087 |
| Al2O3 | 3.813 | 33.676 |
| Fe2O3 | 2.012 | 6.937 |
| CaO | 3.975 | 0.453 |
| MgO | 2.245 | 1.238 |
| K2O | 5.943 | 4.238 |
| Na2O | 0.377 | 0.436 |
2.2. Thermal treatment of china clay
Five samples of china clay were dried in an oven at 105 °C for one hour. The dried clay samples were crushed using a mortar and pestle and sieved to 80 μm. All samples were calcined in a programmable electric furnace for 10 hours with a heating rate of 5 °C min−1 at temperatures of 600, 700, 800, 900 and 1000 °C and were named as C1, C2, C3, C4 and C5 respectively. All the clay samples were stored in air tight plastic bottles to avoid moisture capture.2.3. Synthesis of the geopolymer composites
Different geopolymer mixes were prepared with silica to alumina ratios (SiO2/Al2O3) of 2.5, 2.6, 2.7, 2.8, 2.9, 3, 3.16, 3.28 and 3.5 and the polymers obtained were named as GP1, GP2, GP3, GP4, GP5, GP6, GP7, GP8 and GP9 respectively. The details of the mixes are given in Table 2. For this purpose, china clay thermally activated at the optimum temperature (900 °C) was mixed with bagasse ash and sodium silicate in proper ratios (Table 2). The raw mixes were thoroughly mixed in a laboratory blender and slurries were made using 3 M sodium hydroxide solution. The slurries after thorough mixing were added into a cubic iron mold with an internal width of 50 mm in accordance with ASTM C109 and kept in an oven at 60 °C for 24 hours and then de-molded. The de-molded samples were again kept in an oven at 60 °C for 7 days.| Sample taken | % Weight taken | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bagasse ash | 36.00 | 34.00 | 36.00 | 38.00 | 40.00 | 40.00 | 41.00 | 42.00 | 44.00 |
| China clay | 64.00 | 61.00 | 59.00 | 57.00 | 55.00 | 53.00 | 51.00 | 49.00 | 46.00 |
| Sodium silicate | 0.00 | 5.00 | 5.00 | 5.00 | 5.00 | 7.00 | 8.00 | 9.00 | 10.00 |
| SiO2/Al2O3 | 2.50 | 2.60 | 2.70 | 2.80 | 2.90 | 3.00 | 3.16 | 3.28 | 3.50 |
2.4. Preparation of the geopolymer mortars
Three types of mortar, M1, M2 and M3, with geopolymer to sand ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 respectively were prepared. Cubes of the mortars were prepared in the same way as those of the geopolymer composites and the compressive strength was studied in the same way.
3 respectively were prepared. Cubes of the mortars were prepared in the same way as those of the geopolymer composites and the compressive strength was studied in the same way.
2.5. Characterization of the materials
For characterization of the raw materials, an X-ray fluorescence (XRF) spectrophotometer PW 2582/00 (Philips), an X-ray diffractometer (XRD) JDX-3532 (JEOL Japan) working at 40 kV and 30 mA, a scanning electron microscope (SEM) JSM-5910 (JEOL Japan) working at 20 keV and a Fourier transform infrared (FTIR) spectrometer IR-AFFINITY-1 (SHIMADZU Japan) were used.2.6. Compressive strength of the geopolymer composites and mortars
The compressive strengths of the geopolymer composites and mortars were determined using a Universal Testing Machine (UTM), UTM-100 TON/1000 KN (SHIMADZU Japan), at a curing age of 7 days.3. Results and discussion
3.1. Characterization of the raw materials
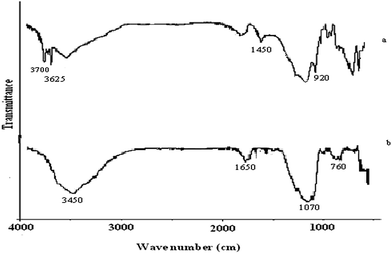
The chemical composition of the bagasse ash as determined by XRF is shown in the Table 1. Bagasse bottom ash contains a silica quantity as high as 78% along with other major components like Al2O3, Fe2O3, CaO and MgO. The sum of silica and alumina in the studied ash is 84% which is in good agreement with the ASTM designation for pozzolana. The loss on ignition (LOI) was recorded as zero, which means that there is no moisture content in the ash and no side reactions taking place on heating.The chemical composition of the china clay shows 46% silica and 33.676% alumina as the major components, while some CaO, MgO and Fe2O3 are also present in comparatively small quantities (Table 1). China clay also exhibits the properties of natural pozzolana as per ASTM. A 14% loss on ignition is due to significant weight loss which corresponds to the loss of water during the conversion of kaolinite into meta-kaolinite.
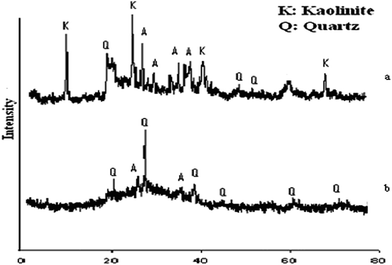
The XRD pattern of bagasse ash is shown in Fig. 1, major diffraction peaks are observed at 25° and 40° indicating the amorphous nature of the silica. Two major peaks are shown in the XRD pattern, i.e. mullite and quartz. The XRD patterns of the uncalcined and calcined clays are presented in Fig. 2a and b respectively. A study of the pattern for the untreated clay shows that all the characteristic peaks are due to the kaolinitic clay and on calcination these peaks were reduced and mostly disappeared (Fig. 2b), showing that the clay becomes amorphous on thermal treatment. The main objective of thermal activation is to convert kaolinite into meta-kaolinite which is confirmed by the XRD pattern of the calcined clay.
3.2. Characterization of the geopolymer
 | ||
| Fig. 3 XRD diffractograms of geopolymers containing (a) clay calcined at 800 °C and (b) clay calcined at 900 °C. | ||
The appearance of peaks for quartz in XRD diffractograms of the geopolymer samples indicates that quartz can’t be dissolved during geopolymerization.
The SEM micrographs of the geopolymers synthesized from clay calcined at 800 and 900 °C with a silica to alumina ratio of 2.7 are shown in Fig. 4a and b respectively. The SEM image of the geopolymer obtained at 800 °C indicates a spongy nature, while that obtained at 900 °C suggests a compact nature indicating that the geopolymer synthesis is more significant when using the clay calcined at 900 °C than that at 800 °C. Some small cracks are seen on the micrographs of the geopolymer matrices. For the SEM analysis, a solid sample of geopolymer was cut into reasonably sized pieces, which were coated with silver to make them conducting. The cracks may have occurred during either cutting or coating with silver, or may be due to some instrumental error. Some unreacted particles can also be identified which may be due to incomplete reactions during geopolymer formation.
 | ||
| Fig. 7 Effect of the calcination temperature of the clay on the compressive strength of the geopolymers. | ||
The ratio of silica to alumina (Si/Al) has a great impact on the compressive strength of the resulting geopolymer composites. The compressive strength of geopolymers having different Si/Al ratios is shown in Fig. 8. Comparing the strengths of the different composites, i.e. GP1, GP2, GP3, GP4, GP5, GP6, GP7, GP8 and GP9, containing china clay calcined at 900 °C (C3) shows that the compressive strength of the geopolymer having a silica to alumina ratio of 2.7, is the highest compared to all other composites, which may be due to the stoichiometric ratio of silica to alumina in the geopolymer, while the lower strengths for the other ratios is attributed to the fact that excess silica affects the bonding between silica and alumina in the geopolymer.
The concentration of sodium hydroxide also plays very important roles in the synthesis and development of the strength of the geopolymer. The effect of sodium hydroxide on the compressive strength of the geopolymer composites is shown in Fig. 9. From the figure it is clear that the highest compressive strength of the geopolymer composites is observed with 3 M NaOH. Further increases in the alkali concentration see the compressive strength going on to decrease. The decrease in strength may be due to the presence of an excess amount of NaOH, above the stoichiometric value, and due to its hygroscopic nature.
The compressive strengths of the geopolymer mortars are given in Table 3. Three types of mortar have been studied with polymer to sand ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. For all these mortars the silica to alumina ratio was kept as 2.7, as this is the optimum ratio for maximum strength. It is clear from the table that the compressive strength decreases with the increasing proportion of sand in the mortar. The highest strength was recorded for the mortar with a 1
3. For all these mortars the silica to alumina ratio was kept as 2.7, as this is the optimum ratio for maximum strength. It is clear from the table that the compressive strength decreases with the increasing proportion of sand in the mortar. The highest strength was recorded for the mortar with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of polymer to sand.
1 ratio of polymer to sand.
| Sample ID | Ratio of composite to sand | Compressive strength (PSI) |
|---|---|---|
| M1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13.41 |
| M2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.70 |
| M3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
6.42 |
4. Conclusion
China clay samples were calcined at different temperatures and used as the aluminosilicate material in the synthesis of geopolymer binders. The effect of calcination temperature on the properties of the geopolymer including setting time and compressive strength was investigated and it was shown that geopolymer pastes obtained from china clay calcined from 600–900 °C showed a drop in their setting times and an increase in their compressive strengths. Above 900 °C, an increase in setting time and a decrease in compressive strength were observed. From these results it is concluded that when the de-hydroxylation of kaolinite clays takes place, disorder in the structure of meta-kaolinite occurs which changes with calcination temperature. This maximum disorder is attained at 900 °C. An increase in the disorder in meta-kaolinite promotes better characteristics of the geopolymer. Above this temperature, the disorder again decreases. Moreover, a silica to alumina ratio of 2.7, and 3 M NaOH were identified as optimum for achieving the highest compressive strength.References
- J. G. S. Jaarsveld and J. S. J. Deventer, Ind. Eng. Chem. Res., 1999, 38, 3929–3941 Search PubMed.
- M. B. Ogundiran and S. Kumar, Appl. Clay Sci., 2015, 108, 173–181 CrossRef CAS PubMed.
- X. Yao, Thermochim. Acta, 2009, 493, 49–54 CrossRef CAS PubMed.
- I. Perna, T. Hanzlícek and M. Supova, Appl. Clay Sci., 2014, 102, 213–219 CrossRef CAS PubMed.
- D. Dimas, L. Giannopoulou and D. Panias, J. Mater. Sci., 2009, 44, 3719–3730 CrossRef CAS.
- S. Alonso and A. Palomo, Cem. Concr. Res., 2001, 31, 25–30 CrossRef CAS.
- Y. J. Zhang, Mater. Sci. Eng., A, 2010, 213–219 CAS.
- N. Amin, Construct. Build. Mater., 2012, 34, 381–384 CrossRef PubMed.
- N. Amin, M. Tahir Shah and K. Ali, Mag. Concr. Res., 2009, 61, 779–785 CrossRef CAS.
- K. Ali, N. Amin and M. Tahir Shah, J. Chem. Soc. Pak., 2009, 31, 375–378 CAS.
- N. Amin, J. Mat. Civ. Eng., 2011, 23, 717–720 CrossRef CAS.
- F. G. M. Aredes, T. M. B. Campos, J. P. B. Machado, K. K. Sakane, G. P. Thim and D. D. Brunelli, Ceram. Interfaces, 2015, 41, 7302–7311 CrossRef CAS PubMed.
- J. Davidovits, J. Therm. Anal., 1991, 37, 1633–1656 CrossRef CAS.
- M. L. Granizo, S. Alonzo, M. T. BlancoVarela and A. Palomo, J. Am. Ceram. Soc., 2002, 85, 225–231 CrossRef CAS PubMed.
- E. Kamseu, C. Leonelli, U. F. Melo, D. Perera and P. N. Lemougna, Interceram, 2011, 60, 25–31 CAS.
- E. Kamseu, C. Leonelli, U. F. Melo, D. Perera and P. N. Lemougna, Interceram, 2011, 60, 25–31 CAS.
- M. Olivia and H. Nikraz, Mater. Des., 2012, 36, 191–198 CrossRef CAS PubMed.
- R. Fernandez, F. Martirena and K. L. Scrivener, Cem. Concr. Res., 2011, 41, 113–122 Search PubMed.
- J. Davidovits and M. Davidovits, Ceram. Eng. Sci. Proc., 1988, 9, 842–853 Search PubMed.
- A. Palomo, Cem. Concr. Res., 1999, 29, 997–1004 CrossRef CAS.
- R. Cioffi, L. Maffucci and L. Santoro, Resour., Conserv. Recycl., 2003, 40, 27–38 CrossRef.
- Y. Zhang and S. Wei, Indian Concr. J., 2006, 1–5 Search PubMed.
- F. Zibouche, H. Kerdjoudj and H. van Damme, Appl. Clay Sci., 2009, 43, 453–458 CrossRef CAS PubMed.
- T. Chareerat, P. Chindaprasirt and V. Sirivivatnanon, Cem. Concr. Compos., 2007, 29, 224–229 CrossRef PubMed.
- H. Xu, J. L. Provis, J. S. J. van and P. V. Deventer, ACI Mater. J., 2008, 105, 131–139 CAS.
- M. Palacios and F. Puertas, Cem. Concr. Res., 2007, 37, 691–702 CrossRef CAS PubMed.
- V. F. F. Barbosa, K. J. D. MacKenzie and C. Thaumaturgo, J. Inorg. Mater., 2000, 309–317 CrossRef CAS.
- V. F. F. Barbosaand and K. J. D. MacKenzie, Mater. Lett., 2003, 57, 1477–1482 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |