One-step method to produce methyl-d-glucoside from lignocellulosic biomass
Abstract
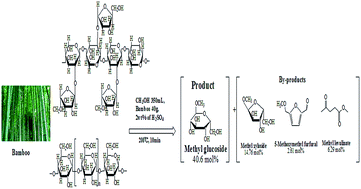
One-pot acid-catalyzed methanolysis was applied to the liquefaction of biomass to obtain a high molar yield of methyl-D-glucoside at moderate temperature in a short time. A high bamboo conversion ratio (85 wt%) of bamboo and high molar yield of methyl-D-glucoside (40.6 mol%) were achieved. The conditions for high yield were methanol/bamboo mass ratio of 7 (350 mL methanol and 40 g bamboo), 2.0 wt% of catalyst, reaction temperature of 200 °C and reaction time of 10 min. Both hemicelluloses and cellulose (holocellulose) in the lignocellulosic biomass can convert into methyl-D-glucoside, a key and stable product. Methanolysis of biomass proved more efficient than its hydrolysis with an acid catalyst under similar reaction conditions, in water glucose yields were reduced to only 2–8 mol%.

 Please wait while we load your content...
Please wait while we load your content...